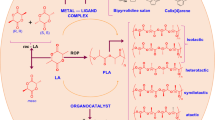
Abstract
Despite the advantages of organocatalysts, the preparation of high-molecular-weight polylactide using organocatalysts remains as a challenge probably due to the transesterification or back-biting side reactions at low catalyst and initiator loadings. In this contribution, a series of organobases and ureas were combined as binary catalysts and screened for the ROP of lactide. The bisurea as the co-catalyst exhibited higher catalytic activity while remaining good controllability over the polymerization compared to the monourea. The controlled ROP of l-lactide was achieved by judiciously chosen of suitable combination of organobase and bisurea. Well-defined polylactides bearing various terminal groups were prepared using different alcohols as initiators. Remarkably, a high-molecular-weight PLA sample with a Mn up to 73.8 kDa was obtained at a low catalyst loading of 0.05 mol% relative to the monomer, which exhibited good mechanical properties that comparable to the commercial PLA.







Similar content being viewed by others
Data availability
The data that support the findings of this study are available in the Supporting Information of this article.
References
Zhang X, Fevre M, Jones GO, Waymouth RM (2018) Catalysis as an Enabling Science for Sustainable Polymers. Chem Rev 118:839–885
Plastics – the Facts (2021) PlasticsEurope: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021/. Accessed 18 May 2023
Hong M, Chen EYX (2017) Chemically recyclable polymers: a circular economy approach to sustainability. Green Chem 19:3692–3706
Fagnani DE, Tami JL, Copley G, Clemons MN, Getzler YDYL, McNeil AJ (2020) 100th Anniversary of Macromolecular Science Viewpoint: Redefining Sustainable Polymers. ACS Macro Lett 10:41–53
Worch JC, Dove AP (2020) 100th Anniversary of Macromolecular Science Viewpoint: Toward Catalytic Chemical Recycling of Waste (and Future) Plastics. ACS Macro Lett 9:1494–1506
Haider TP, Völker C, Kramm J, Landfester K, Wurm FR (2019) Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew Chem Int Ed 58:50–62
Tang X, Chen EYX (2019) Toward Infinitely Recyclable Plastics Derived from Renewable Cyclic Esters. Chem 5:284–312
Haque FM, Ishibashi JSA, Lidston CAL, Shao H, Bates FS, Chang AB, Coates GW, Cramer CJ, Dauenhauer PJ, Dichtel WR, Ellison CJ, Gormong EA, Hamachi LS, Hoye TR, ** M, Kalow JA, Kim HJ, Kumar G, LaSalle CJ, Liffland S, Lipinski BM, Pang Y, Parveen R, Peng X, Popowski Y, Prebihalo EA, Reddi Y, Reineke TM, Sheppard DT, Swartz JL, Tolman WB, Vlaisavljevich B, Wissinger J, Xu S, Hillmyer MA (2022) Defining the Macromolecules of Tomorrow through Synergistic Sustainable Polymer Research. Chem Rev 122:6322–6373
Anastas PT (2019) Beyond Reductionist Thinking in Chemistry for Sustainability. Trends Chem 1:145–148
Oh JK (2011) Polylactide (PLA)-based amphiphilic block copolymers: synthesis, self-assembly, and biomedical applications. Soft Matter 7:5096–5108
Huang Y, ** Y, Wang B, Tian H, Weng Y, Men S (2022) Compatibilization and Toughening of Biodegradable Polylactic Acid/Cellulose Acetate Films by Polyamide Amine Dendrimers. J Polym Environ 30:1758–1771
Huang Y, ** Y, Wang B, Tian H, Weng Y, Sun K, Men S (2022) Preparation and characterization of compatibilized and toughened polylactic acid/cellulose acetate films by long-chain hyperbranched polymers. J Appl Polym Sci 139
Sun J, Huang Y, ** Y, Tian H, Men S (2022) Improvement of mechanical properties and heat distortion temperature of polylactic acid by highly aromatic hyperbranched polyamide. J Appl Polym Sci 139
Shen Y, Li ZB (2020) Ring-opening Polymerization of Cyclic Esters by Utilizing Organophosphazene Bases toward Biodegradable Polyesters. Acta Polym Sin 51:777–790
Kamber NE, Jeong W, Waymouth RM, Pratt RC, Lohmeijer BGG, Hedrick JL (2007) Organocatalytic Ring-Opening Polymerization. Chem Rev 107:5813–5840
Kiesewetter MK, Shin EJ, Hedrick JL, Waymouth RM (2010) Organocatalysis: Opportunities and Challenges for Polymer Synthesis. Macromolecules 43:2093–2107
Mezzasalma L, Harrisson S, Saba S, Loyer P, Coulembier O, Taton D (2019) Bulk Organocatalytic Synthetic Access to Statistical Copolyesters from L-Lactide and ε-Caprolactone Using Benzoic Acid. Biomacromolecules 20:1965–1974
Makiguchi K, Yamanaka T, Kakuchi T, Terada M, Satoh T (2014) Binaphthol-derived phosphoric acids as efficient chiral organocatalysts for the enantiomer-selective polymerization of rac-lactide. Chem Commun 50:2883–2885
Dove AP, Li H, Pratt RC, Lohmeijer BG, Culkin DA, Waymouth RM, Hedrick JL (2006) Stereoselective polymerization of rac- and meso-lactide catalyzed by sterically encumbered N-heterocyclic carbenes. Chem Commun 2881–2883
Li H, Ai B-R, Hong M (2017) Stereoselective ring-opening polymerization of rac-lactide by bulky chiral and achiral N-heterocyclic carbenes. Chin J Polym Sci 36:231–236
Lohmeijer BGG, Pratt RC, Leibfarth F, Logan JW, Long DA, Dove AP, Nederberg F, Choi J, Wade C, Waymouth RM, Hedrick JL (2006) Guanidine and Amidine Organocatalysts for Ring-Opening Polymerization of Cyclic Esters. Macromolecules 39:8574–8583
Liu SF, Li HK, Zhao N, Li ZB (2018) Stereoselective Ring-Opening Polymerization of rac-Lactide Using Organocatalytic Cyclic Trimeric Phosphazene Base. ACS Macro Lett 7:624–628
Zhang L, Nederberg F, Messman JM, Pratt RC, Hedrick JL, Wade CG (2007) Organocatalytic stereoselective ring-opening polymerization of lactide with dimeric phosphazene bases. J Am Chem Soc 129:12610–12611
Jiang ZL, Zhao JP, Zhang GZ (2019) Ionic Organocatalyst with a Urea Anion and Tetra-n-butyl Ammonium Cation for Rapid, Selective, and Versatile Ring-Opening Polymerization of Lactide. ACS Macro Lett 8:759–765
Kou XH, Shen Y, Li ZB (2020) Stereoselective Ring-opening Polymerization of rac-Lactide Using Chiral Urea/Strong Organobase Binary Catalyst System. Acta Polym Sin 51:1121–1129
Lin B, Waymouth RM (2017) Urea Anions: Simple, Fast, and Selective Catalysts for Ring-Opening Polymerizations. J Am Chem Soc 139:1645–1652
Zhang XY, Jones GO, Hedrick JL, Waymouth RM (2016) Fast and selective ring-opening polymerizations by alkoxides and thioureas. Nat Chem 8:1047–1053
Lin BH, Waymouth RM (2018) Organic Ring-Opening Polymerization Catalysts: Reactivity Control by Balancing Acidity. Macromolecules 51:2932–2938
Pothupitiya JU, Dharmaratne NU, Jouaneh TMM, Fastnacht KV, Coderre DN, Kiesewetter MK (2017) H-Bonding Organocatalysts for the Living, Solvent-Free Ring-Opening Polymerization of Lactones: Toward an All-Lactones. All-Conditions Approach Macromolecules 50:8948–8954
Orhan B, Tschan MJL, Wirotius A-L, Dove AP, Coulembier O, Taton D (2018) Isoselective Ring-Opening Polymerization of rac-Lactide from Chiral Takemoto’s Organocatalysts: Elucidation of Stereocontrol. ACS Macro Lett 7:1413–1419
Sanchez-Sanchez A, Rivilla I, Agirre M, Basterretxea A, Etxeberria A, Veloso A, Sardon H, Mecerreyes D, Cossío FP (2017) Enantioselective Ring-Opening Polymerization of rac-Lactide Dictated by Densely Substituted Amino Acids. J Am Chem Soc 139:4805–4814
Liu Y, Zhang J, Kou X, Liu S, Li Z (2022) Highly Active Organocatalysts for Stereoselective Ring-Opening Polymerization of Racemic Lactide at Room Temperature. ACS Macro Lett 11:1183–1189
Zaky MS, Wirotius AL, Coulembier O, Guichard G, Taton D (2021) A chiral thiourea and a phosphazene for fast and stereoselective organocatalytic ring-opening-polymerization of racemic lactide. Chem Commun 57:3777–3780
Zaky MS, Wirotius A-L, Coulembier O, Guichard G, Taton D (2022) Reaching High Stereoselectivity and Activity in Organocatalyzed Ring-Opening Polymerization of Racemic Lactide by the Combined Use of a Chiral (Thio)Urea and a N-Heterocyclic Carbene. ACS Macro Lett 11:1148–1155
Fastnacht KV, Spink SS, Dharmaratne NU, Pothupitiya JU, Datta PP, Kiesewetter ET, Kiesewetter MK (2016) Bis- and Tris-Urea H-Bond Donors for Ring-Opening Polymerization: Unprecedented Activity and Control from an Organocatalyst. ACS Macro Lett 5:982–986
Hewawasam RS, Kalana ULDI, Dharmaratne NU, Wright TJ, Bannin TJ, Kiesewetter ET, Kiesewetter MK (2019) Bisurea and Bisthiourea H-Bonding Organocatalysts for Ring-Opening Polymerization: Cues for the Catalyst Design. Macromolecules 52:9232–9237
Spink SS, Kazakov OI, Kiesewetter ET, Kiesewetter MK (2015) Rate Accelerated Organocatalytic Ring-Opening Polymerization of L-Lactide via the Application of a Bis(thiourea) H-bond Donating Cocatalyst. Macromolecules 48:6127–6131
Li C, Wang L, Yan Q, Liu F, Shen Y, Li Z (2022) Rapid and Controlled Polymerization of Bio-sourced δ-Caprolactone toward Fully Recyclable Polyesters and Thermoplastic Elastomers. Angew Chem Int Ed 61
Li J, Liu F, Liu Y, Shen Y, Li Z (2022) Functionalizable and Chemically Recyclable Thermoplastics from Chemoselective Ring-Opening Polymerization of Bio-renewable Bifunctional α-Methylene-δ-valerolactone. Angew Chem Int Ed 61
Coderre DN, Fastnacht KV, Wright TJ, Dharmaratne NU, Kiesewetter MK (2018) H-Bonding Organocatalysts for Ring-Opening Polymerization at Elevated Temperatures. Macromolecules 51:10121–10126
Pothupitiya JU, Hewawasam RS, Kiesewetter MK (2018) Urea and Thiourea H-Bond Donating Catalysts for Ring-Opening Polymerization: Mechanistic Insights via (Non)linear Free Energy Relationships. Macromolecules 51:3203–3211
McCutcheon CJ, Zhao B, Ellison CJ, Bates FS (2021) Crazing and Toughness in Diblock Copolymer-Modified Semicrystalline Poly(L-lactide). Macromolecules 54:11154–11169
Acknowledgment
The authors appreciate financial support by National Natural Science Foundation of China (No. 22075160), Taishan Scholar Foundation of Shandong Province (No. tsqn202103078).
Author information
Authors and Affiliations
Contributions
Wei Zhou: Formal analysis, Investigation, Writing - Original Draft. Chen Xu and Yalei Liu: Validation.Yong Shen: Conceptualization, Methodology, Writing - Review & Editing, Supervision, Funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, W., Xu, C., Liu, Y. et al. Preparation of high-molecular-weight polylactide by ring-opening polymerization of l-lactide using base/bisurea binary organocatalyst. J Polym Res 30, 230 (2023). https://doi.org/10.1007/s10965-023-03621-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-023-03621-w