Abstract
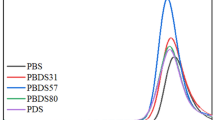
Poly(butylene succinate) (PBS), a biodegradable material, has excellent mechanical properties and processing properties. Brassylic acid (BA) is a kind of diacid with long chains derived from biomass. In this study, poly(butylene succinate-co-butylene brassylate) is synthesized to investigate the effects of BA on the structure and properties of PBS. The relationship between the melting temperature of copolyesters and the content of butylene brassylate units is V-shaped, which is typical of isodimorphic random copolyesters. Due to the introduction of BA, the density of unstable ester bonds in copolyesters gradually decreases and the initial thermal decomposition temperature of copolyesters gradually increases with the increase of BA. The dynamic mechanical analysis showed that the glass transition temperature of PBS was reduced by the introduction of BA. When the BA content is 25%, the copolyesters and PBS have the same crystal structure. When the BA content is 50–100%, the copolyesters and poly(butylene brassylate) have the same crystal structure, which indicates that the butylene brassylate units are easier to crystallize than the butylene succinate units. The ductility of copolyesters increases with the addition of BA. When the BA content is 50%, the elongation at break reaches the maximum (> 2000%). The copolyesters are suited for films due to high elongation at break.









Similar content being viewed by others
Data Availability
The data that support the findings of this study are available on request from the corresponding author upon reasonable request.
References
Bougarech A, Abid M, Abid S, Fleury E (2016) Synthesis, characterization and thermal, hydrolytic and oxidative degradation study of biobased (BisFuranic-Pyridinic) copolyesters. Polym Degrad Stab 133:283–292
Chebbi Y, Kasmi N, Majdoub M, Cerruti P, Scarinzi G, Malinconico M, Dal Poggetto G, Papageorgiou GZ, Bikiaris DN (2019) Synthesis, characterization, and biodegradability of novel fully biobased poly(decamethylene-co-isosorbide 2,5-furandicarboxylate) copolyesters with enhanced mechanical properties. ACS Sustain Chem Eng 7(5):5501–5514
Hu H, Zhang R, Wang J, Ying WB, Zhu J (2018) Fully bio-based poly(propylene succinate-co-propylene furandicarboxylate) copolyesters with proper mechanical, degradation and barrier properties for green packaging applications. Eur Polymer J 102:101–110
Ignaczak W, Sobolewski P, El Fray M (2019) Bio-based PBT–DLA copolyester as an alternative compatibilizer of PP/PBT blends. Polymers. https://doi.org/10.3390/polym11091421
Sonseca A, McClain A, Puskas JE, El Fray M (2019) Kinetic studies of biocatalyzed copolyesters of poly(butylene succinate) (PBS) containing fully bio-based dilinoleic diol. Eur Polymer J 116:515–525
**e H, Wu L, Li B-G, Dubois P (2018) Biobased poly(ethylene-co-hexamethylene 2,5-furandicarboxylate) (PEHF) copolyesters with superior tensile properties. Ind Eng Chem Res 57(39):13094–13102
Paszkiewicz S, Walkowiak K, Irska I, Mechowska S, Stankiewicz K, Zubkiewicz A, Piesowicz E, Miadlicki P (2023) Influence of the multiple injection moulding and composting time on the properties of selected packaging and furan-based polyesters. J Polym Environ 31(2):722–742
Silverwood L, Mottoul M, Dumont M-J (2024) A review of end-of-life pathways for poly(ethylene furanoate) and its derivatives. J Polym Environ. https://doi.org/10.1007/s10924-024-03229-1
Kaseem M (2019) Poly(lactic acid) composites. Materials 12:3586
Punet X, Levato R, Bataille I, Letourneur D, Engel E, Mateos-Timoneda MA (2017) Polylactic acid organogel as versatile scaffolding technique. Polymer 113:81–91
Verginio GEA, Montanheiro TLDA, Montagna LS, Marini J, Passador FR (2021) Effectiveness of the preparation of maleic anhydride grafted poly (lactic acid) by reactive processing for poly (lactic acid)/carbon nanotubes nanocomposites. J Appl Polym Sci 138(12):50087
Long Y, Zhang RY, Huang JC, Wang JG, Zhang JW, Rayand N, Hu GH, Yang J, Zhu J (2017) Retroreflection in binary bio-based PLA/PBF blends. Polymer 125:138–143
Anjali M, Sukumar C, Kanakalakshmi A, Shanthi K (2014) Enhancement of growth and production of polyhydroxyalkanoates by Bacillus subtilis from agro-industrial waste as carbon substrates. Compos Interfaces 21(2):111–119
Bhatia SK, Kim J-H, Kim M-S, Kim J, Hong JW, Hong YG, Kim H-J, Jeon J-M, Kim S-H, Ahn J, Lee H, Yang Y-H (2018) Production of (3-hydroxybutyrate-co-3-hydroxyhexanoate) copolymer from coffee waste oil using engineered Ralstonia eutropha. Bioprocess Biosyst Eng 41(2):229–235
Burniol-Figols A, Varrone C, Daugaard AE, Le SB, Skiadas IV, Gavala HN (2018) Polyhydroxyalkanoates (PHA) production from fermented crude glycerol: study on the conversion of 1,3-propanediol to PHA in mixed microbial consortia. Water Res 128:255–266
Liao Q, Guo L, Ran Y, Gao M, She Z, Zhao Y, Liu Y (2018) Optimization of polyhydroxyalkanoates (PHA) synthesis with heat pretreated waste sludge. Waste Manag 82:15–25
Zhang J, Shishatskaya EI, Volova TG, da Silva LF, Chen G-Q (2018) Polyhydroxyalkanoates (PHA) for therapeutic applications. Mater Sci Eng C 86:144–150
Chen S, **ao M, Sun L, Meng Y (2018) Study on thermal decomposition behaviors of terpolymers of carbon dioxide, propylene oxide, and cyclohexene oxide. Int J Mol Sci. https://doi.org/10.3390/ijms19123723
Liang Z-Z, Li X, Hu C-Y, Duan R-L, Wang X-H, Pang X, Chen X-S (2022) Copolymerization of PO/CO2 and lactide by a dinuclear salen-Cr(III) complex: gradient and random copolymers with modificable microstructure. Chin J Polym Sci 40(9):1028–1033
Lin S, Yu W, Wang X, Zhou C (2014) Study on the thermal degradation kinetics of biodegradable poly(propylene carbonate) during melt processing by population balance model and rheology. Ind Eng Chem Res 53(48):18411–18419
**an W, Song L, Liu B, Ding H, Li Z, Cheng M, Ma L (2018) Rheological and mechanical properties of thermoplastic polyurethane elastomer derived from CO2 copolymer diol. J Appl Polym Sci 135(11):45974
Zhang X, Ji G, Yang J, Jiang J, He J, Li T, Huang J, Chen M, Dong W (2023) A green method for preparing mechanically robust poly(propylene carbonate) with full biodegradability via incorporating hybrid natural filler. J Appl Polym Sci 140(19):e53838
Barletta M, Aversa C, Ayyoob M, Gisario A, Hamad K, Mehrpouya M, Vahabi H (2022) Poly(butylene succinate) (PBS): materials, processing, and industrial applications. Prog Polym Sci 132:101579
Di Lorenzo ML, Androsch R, Righetti MC (2017) Low-temperature crystallization of poly(butylene succinate). Eur Polymer J 94:384–391
Liu T-Y, Huang D, Xu P-Y, Lu B, Zhen Z-C, Zheng W-Z, Li X, Wang G-X, Ji J (2022) Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters. e-Polymers 22(1):615–626
Zhang Q, Song M, Xu Y, Wang W, Wang Z, Zhang L (2021) Bio-based polyesters: recent progress and future prospects. Prog Polym Sci 120:101430
Chen TTD, Carrodeguas LP, Sulley GS, Gregory GL, Williams CK (2020) Bio-based and degradable block polyester pressure-sensitive adhesives. Angew Chem Int Ed 59(52):23450–23455
de Oliveira MA, Guimarães Carvalho Machado M, Dias Silva SE, Leite Nascimento T, Martins Lima E, Pound-Lana G, Mosqueira VCF (2019) IR780-polymer conjugates for stable near-infrared labeling of biodegradable polyester-based nanocarriers. Eur Polym J 120:109255
El Mahdi A, M’Sahel M, Medimagh R (2017) Catalyst-free ring opening synthesis of biodegradable poly(ester-urethane)s using isosorbide bio-based initiator. Macromol Chem Phys 218(19):1700077
Rydz J, Sikorska W, Kyulavska M, Christova D (2015) Polyester-based (bio)degradable polymers as environmentally friendly materials for sustainable development. Int J Mol Sci 16:564–596
Ishii M, Okazaki M, Shibasaki Y, Ueda M, Teranishi T (2001) Convenient synthesis of aliphatic polyesters by distannoxane-catalyzed polycondensation. Biomacromol 2(4):1267–1270
Lindström A, Albertsson A-C, Hakkarainen M (2004) Quantitative determination of degradation products an effective means to study early stages of degradation in linear and branched poly(butylene adipate) and poly(butylene succinate). Polym Degrad Stab 83(3):487–493
Yang J, Hao Q, Liu X, Ba C, Cao A (2004) Novel biodegradable aliphatic poly(butylene succinate-co-cyclic carbonate)s with functionalizable carbonate building blocks. 1. Chemical synthesis and their structural and physical characterization. Biomacromolecules 5(1):209–218
Zhu Q-Y, He Y-S, Zeng J-B, Huang Q, Wang Y-Z (2011) Synthesis and characterization of a novel multiblock copolyester containing poly(ethylene succinate) and poly(butylene succinate). Mater Chem Phys 130(3):943–949
Mincheva R, Delangre A, Raquez J-M, Narayan R, Dubois P (2013) Biobased polyesters with composition-dependent thermomechanical properties: synthesis and characterization of poly(butylene succinate-co-butylene azelate). Biomacromolecules 14(3):890–899
Jiang X, Yu Y, Guan Y, Liu T, Pang C, Ma J, Gao H (2020) Random and multiblock PBS copolyesters based on a rigid diol derived from naturally occurring camphor: influence of chemical microstructure on thermal and mechanical properties. ACS Sustain Chem Eng 8(9):3626–3636
Genovese L, Lotti N, Gazzano M, Siracusa V, Dalla Rosa M, Munari A (2016) Novel biodegradable aliphatic copolyesters based on poly(butylene succinate) containing thioether-linkages for sustainable food packaging applications. Polym Degrad Stab 132:191–201
Witt T, Häußler M, Kulpa S, Mecking S (2017) Chain multiplication of fatty acids to precise telechelic polyethylene. Angew Chem Int Ed 56(26):7589–7594
Pérez-Camargo RA, Arandia I, Safari M, Cavallo D, Lotti N, Soccio M, Müller AJ (2018) Crystallization of isodimorphic aliphatic random copolyesters: pseudo-eutectic behavior and double-crystalline materials. Eur Polym J 101:233–247
Yu Y, Sang L, Wei Z, Leng X, Li Y (2017) Unique isodimorphism and isomorphism behaviors of even-odd poly(hexamethylene dicarboxylate) aliphatic copolyesters. Polymer 115:106–117
Kong X, Qi H, Curtis JM (2014) Synthesis and characterization of high-molecular weight aliphatic polyesters from monomers derived from renewable resources. J Appl Polym Sci. https://doi.org/10.1002/app.40579
Ronkay F, Czigány T (2006) Cavity formation and stress-oscillation during the tensile test of injection molded specimens made of PET. Polym Bull 57(6):989–998
Seggiani M, Gigante V, Cinelli P, Coltelli M-B, Sandroni M, Anguillesi I, Lazzeri A (2019) Processing and mechanical performances of poly(butylene succinate–co–adipate) (PBSA) and raw hydrolyzed collagen (HC) thermoplastic blends. Polym Test 77:105900
Safari M, Otaegi I, Aramburu N, Guerrica-Echevarria G, de Ilarduya AM, Sardon H, Müller AJ (2021) Synthesis, structure, crystallization and mechanical properties of isodimorphic PBS-ran-PCL copolyesters. Polymers 13:2263
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
Guoqiang Wang: Data curation, Writing-Original draft preparation, Reviewing and editing, Supervision. Yunfeng Hui: Data curation, Writing-Original draft preparation, Reviewing and editing. Deyu Wei: Data curation. Yueying Wang, Yiheng Yu, Longqing Shi, and Mengke Zhang: Validation, Investigation. **g Hu: Reviewing and editing, Investigation.
Corresponding authors
Ethics declarations
Competing Interests
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, G., Hui, Y., Wei, D. et al. Poly(butylene Succinate-co-butylene Brassylate) Derived from Brassylic Acid: Structures and Properties. J Polym Environ (2024). https://doi.org/10.1007/s10924-024-03330-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s10924-024-03330-5