Abstract
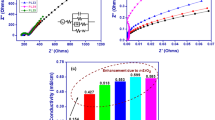
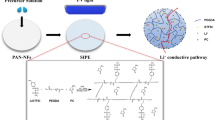
Flexible polymer electrolytes with efficient electrochemical characteristics were necessary to meet the requirements of smart electronics technology and foldable electronics. It is both important and difficult to design and construct a flexible lithium polymer electrolyte with higher ionic conductivity and better dielectric properties for use in electrochemical storage devices because of the need to increase storage capacity and electrochemical stability. This research describes the inclusion of Nickel oxide nanoparticles (n-NiO), which are environmentally benign, doped lithium flexible polymer electrolytes (n-FLPEs), via the solution-cast method with polymer matrices of poly (vinylidene fluoride-hexafluoropropylene) (PVdF-HFP), poly (ethylene oxide) (PEO), which is a biodegradable polymer, as well as Lithium trifluoromethanesulfonate (LiCF3SO3). When n-NiO is incorporated into FLPEs, the ionic conductivity increases by a factor of nearly four and a half. The εʹmax value increased four times, and ε″max value increased seventeen times owing to n-NiO inclusion. We used cyclic voltammetry (CV) and chronoamperometry (CA) to study the charge carrier buildup, ionic strength, and transference number in the optimal n-FLPE membrane. n-FLPEs have been shown to be electrochemically stable and capable of transporting ions due to chemical shifts in functional groups illustrated by X-ray photoelectron spectroscopy (XPS) investigations. The significance of the [υs(SO3)] mode in ionic conductivity has been extensively addressed. We analysed the FESD's discharging behaviour at a 0.04 C assessment, or an average current during discharge of 5.128 mA over 25 h.















Similar content being viewed by others
Data Availability
The authors affirm that the paper has the information needed to support the study's results.
References
Gautam KP, Acharya D, Bhatta I, Subedi V, Das M, Neupane S, Kunwar J, Chhetri K, Yadav AP (2022) Nickel oxide-incorporated polyaniline nanocomposites as an efficient electrode material for supercapacitor application. Inorganics 10(6):86. https://doi.org/10.3390/inorganics10060086
Meng Q, Cai K, Chen Y, Chen L (2017) Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 36:268–285. https://doi.org/10.1016/j.nanoen.2017.04.040
Hu L, Wu H, Mantia FL, Yang Y, Cui Y (2010) Thin, flexible secondary Li-ion paper batteries. ACS Nano 4:5843–5848. https://doi.org/10.1021/nn1018158
Yu X, Boyer MJ, Hwang GS, Manthiram A (2018) Room-temperature aluminum-sulfur batteries with a lithium-ion-mediated ionic liquid electrolyte. Chemistry 4:586–598. https://doi.org/10.1016/j.chempr.2017.12.029
Osiak M, Geaney H, Armstrong E, O’Dwyer C (2014) Structuring materials for lithium-ion batteries: advancements in nanomaterial structure, composition, and defined assembly on cell performance. J Mater Chem A 2:9433. https://doi.org/10.1039/C4TA00534A
Shanthi PM, Hanumantha PJ, Albuquerque T, Gattu B, Kumta PN (2018) Novel composite polymer electrolytes of PVdF-HFP derived by electrospinning with enhanced Li-ion conductivities for rechargeable lithium–sulfur batteries. ACS Appl Energy Mater 1(2):483–494. https://doi.org/10.1021/acsaem.7b00094
Bass PS, Zhang L, Tu M, Cheng Z (2018) Enhancement of biodegradable poly (ethylene oxide) ionic–polymer metallic composite actuators with nanocrystalline cellulose fillers. Actuators 7(4):72. https://doi.org/10.3390/act7040072
Thomas S, GrohensJyotishkumar Y (2015) Characterization of polymer blends: miscibility, morphology, and interfaces, 1st edn. Wiley, New York
Sim LN, Majid SR, Arof AK (2012) FTIR studies of PEMA/PVdF-HFP blend polymer electrolyte system incorporated with LiCF3SO3 salt. Vib Spectrosc 58:57–66. https://doi.org/10.1016/j.vibspec.2011.11.005
Alipour M, Ziebert C, Conte FV, Kizilel R (2020) A review on temperature-dependent electrochemical properties, aging, and performance of lithium-ion cells. Batteries 6:35. https://doi.org/10.3390/batteries6030035
Negar Z, Khanmirzaei MH, Ramesh S, Ramesh K (2017) Presence of NaI in PEO/PVdF-HFP blend based gel polymer electrolytes for fabrication of dye-sensitized solar cells. Mater Sci Semicond Process 66:144–148. https://doi.org/10.1016/j.mssp.2017.04.016
Mouraliraman D, Shaji N, Praveen S, Nanthagopal M, Ho CW, Karthik MV, Kim T, Lee CW (2022) Thermally stable PVDF-HFP-based gel polymer electrolytes for high-performance lithium-ion batteries. Nanomaterials 12:1056. https://doi.org/10.3390/nano12071056
Yesappa L, Kumar SPA, Vijeth H, Basappa M, Sanjeev G, Devendrappa H (2019) Effect of electron beam irradiation on structure, morphology, and optical properties of PVDF-HFP/PEO blend polymer electrolyte films. J Radioanal Nucl Chem 322:05–10. https://doi.org/10.10907/s06466-0
Anil A, Sharma AL (2017) Structural, microstructural and electrochemical properties of dispersed-type polymer nanocomposite films. J Phys D 50:443002. https://doi.org/10.1088/1361-6463/aa9f69
Reddy MR, Subrahmanyam AR, Reddy MM, Kumar JS, Kamalaker V, Reddy MJ (2016) X-RD, SEM, FT-IR, DSC studies of polymer blend films of PMMA and PEO. Mater Today 3(10):3713–3718. https://doi.org/10.1016/j.matpr.2016.11.018
Danquah SA, Strimaitis J, Denize CF, Pradhan SK, Bahoura M (2022) LLCZN/PEO/LiPF6 composite solid-state electrolyte for safe energy storage application. Batteries 8:3. https://doi.org/10.3390/batteries8010003
Dhatarwal P, Sengwa RJ (2020) Synergistic effects of salt concentration and polymer blend composition on the crystal phases, dielectric relaxation, and ion conduction in PVDF/PEO/LiCF3SO3 solid polymer electrolytes. Ionics 26:2259–2275. https://doi.org/10.1007/s11581-019-03337-2
Jhuo HJ, Sunil S, Chen HL, Chen SA (2018) A nonvolatile morphology regulator for enhancing the molecular order in the active layer and power conversion efficiency of polymer solar cells. J Mater Chem A 6(19):8874–8879. https://doi.org/10.1039/C8TA01739E
Cheng YJ, Yang SH, Hsu CS (2009) Synthesis of conjugated polymers for organic solar cell applications. Chem Rev 109(11):5868–5923. https://doi.org/10.1021/cr900182s
Chapi S (2016) Enhanced electrochemical, structural, optical, thermal stability and ionic conductivity of (PEO/PVP) polymer blend electrolyte for electrochemical applications. Ionics 22(6):803–814. https://doi.org/10.1007/s11581-015-1600-2
Chapi S (2020) Structural and electrochemical properties of polymer blend based ZnO nanocomposite solid polymer electrolytes by spin–coating method. J Nano-Electron Phys 12(2):02043(1)-02043(5)
Chapi S (2021) Influence of Co2+ on the structure, conductivity, and electrochemical stability of poly (ethylene oxide)-based solid polymer electrolytes: energy storage devices. J Electron Mater 50(3):1558–1571. https://doi.org/10.1007/s11664-020-08706-6
Prasanna K, Subburaj T, Lee WJ, Lee CW (2014) Polyethylene separator: stretched and coated with porous nickel oxide nanoparticles for enhancement of its efficiency in Li-ion batteries. Electrochim Acta 137:273–279. https://doi.org/10.1016/j.electacta.2014.06.026
Yue GH, Zhao YC, Wang CG, Zhang XX, Zhang XQ, **e QS (2015) Flower-like nickel oxide nanocomposites anode materials for excellent performance lithium-ion batteries. Electrochim Acta 152:315–322. https://doi.org/10.1016/j.electacta.2014.11.177
Shaalan NM, Hanafy TA, Rashad M (2021) Dual optical properties of NiO-doped PVA nanocomposite films. Opt Mater 119:111325. https://doi.org/10.1016/j.optmat.2021.111325
Mettu MR, Mallikarjun A, Reddy MV, Reddy MJ, Kumar JS (2022) Investigation of structural and optical properties of PMMA/PVdF-HFP polymer blend system. Proc Fourth Int Conf Invent Mater Sci Appl. https://doi.org/10.1007/978-981-16-4321-7_26
Jeedi VR, Narsaiah EL, Yalla M, Swarnalatha R, Reddy SN, Chary AS (2020) Structural and electrical studies of PMMA and PVdF based blend polymer electrolyte. SN Appl Sci 2:2093. https://doi.org/10.1007/s42452-020-03868-8
Ganta KK, Jeedi VR, Kumar KV, Narsaiah EL (2021) Preparation, characterization and impedance spectroscopic studies of Na+ ion conducting PEO+ PVDF-blended polymer electrolytes. Int J Polym Anal Charact 26:130–144. https://doi.org/10.1080/1023666X.2020.1860396
Sharma S, Pathak D, Dhiman N, Kumar R (2017) Characterization of PVdF-HFP-based nanocomposite plasticized polymer electrolytes. Surf Innov. https://doi.org/10.1680/jsuin.17.00019
Aziz SB, Brevik I, Hamsan MH, Brza MA, M. Nofal M, Abdullah AM, Rostam S, Al-Zangana S, Muzakir SK, Kadir MF (2020) Compatible solid polymer electrolyte based on methyl cellulose for energy storage application: structural, electrical, and electrochemical properties. Polymers 12:2257. https://doi.org/10.3390/polym12102257
Brza MA, Aziz SB, Anuar H, Ali F, Hamsan MH, Kadir MF, Abdulwahid RT (2020) Metal framework as a novel approach for the fabrication of electric double layer capacitor device with high energy density using plasticized poly (vinyl alcohol): ammonium thiocyanate based polymer electrolyte. Arab J Chem 13:7247–7263. https://doi.org/10.1016/j.arabjc.2020.08.006
Ramesh S, Wen LC (2010) Investigation on the effects of addition of SiO2 nanoparticles on ionic conductivity, FTIR, and thermal properties of nanocomposite PMMA–LiCF3SO3–SiO2. Ionics 16:255–262. https://doi.org/10.1007/s11581-009-0388-3
Alias AN, Kudin TI, Zabidi ZM, Harun MK, Yahya MZ (2012) Effect of salt concentration and humidity on the ionic conductivity of poly (vinylidene fluoride–hexafluoropropylene) (PVdF-HFP). Adv Mater Res 501:39–43. https://doi.org/10.4028/www.scientific.net/AMR.501.39
Sengwa RJ, Dhatarwal P, Choudhary S (2014) Role of preparation methods on the structural and dielectric properties of plasticized polymer blend electrolytes: correlation between ionic conductivity and dielectric parameters. Electrochim Acta 142:359–370. https://doi.org/10.1016/j.electacta.2014.07.120
Kingslin Mary Genova F, Selvasekarapandian S, Vijaya N, Sivadevi S, Premalatha M, Karthikeyan S (2017) Lithium ion-conducting polymer electrolytes based on PVA–PAN doped with lithium triflate. Ionics 23:2727–2734. https://doi.org/10.1007/s11581-017-2052-7
Sampath Kumar L, Christopher Selvin P, Selvasekarapandian S (2021) Impact of lithium triflate (LiCF3SO3) salt on tamarind seed polysaccharide-based natural solid polymer electrolyte for application in electrochemical device. Polym Bull 78:1797–1819. https://doi.org/10.1007/s00289-020-03185-5
Ruan Z, Du Y, Pan H, Zhang R, Zhang F, Tang H, Zhang H (2022) Incorporation of poly (ionic liquid) with PVDF-HFP-based polymer electrolyte for all-solid-state lithium-ion batteries. Polymers 14:1950. https://doi.org/10.3390/polym14101950
Ajay Kumar P, Mallikarjun A, Mettu MR, Anand Kumar Sagar P, Thirmal C, Jaipal Reddy M, Siva Kumar J (2023) Investigation of flexible electrochemical storage with Li+/PVdF-HFP/PEO blend. J Polym-Plast Tech Mater 62(10):1205–1222. https://doi.org/10.1080/25740881.2023.2204904
Ajay Kumar P, Mallikarjun A, Mettu MR, Jaipal Reddy M, Siva Kumar J (2023) Effective nanocomposite flexible electrochemical storage with Li+/PVdF-HFP/PEO/n-ZrO2 complex. J Polym Res 30(6):1–13. https://doi.org/10.1007/s10965-023-03571-3
Pecovska-Gjorgjevich M, Aleksovska S, Dimitrovska-Lazova S, Marinšek M (2016) The role of Cr/Co substitution on dielectric properties of gadolinium orthochromite. Phys Scr 91:045805. https://doi.org/10.1088/0031-8949/91/4/045805
Gondivaraj G, Baskaran N, Shahi K, Monoravi P (1995) Preparation, conductivity, complex permittivity and electric modulus in AgI-Ag2O-SeO3-MoO3 glasses. Solid State Ion 76:47–55. https://doi.org/10.1016/0167-2738(94)00204-6
Singh KP, Gupta PN (1998) Study of dielectric relaxation in polymer electrolytes. Eur Polym J 34:1023–1029. https://doi.org/10.1016/S0014-3057(97)00207-3
Hadi JM, Aziz SB, Saeed S, Brza MA, Abdulwahid RT, Hamsan MH, Abdullah R, Kadir MF, Muzakir SK (2020) Investigation of ion transport parameters and electrochemical performance of plasticized biocompatible chitosan-based proton conducting polymer composite electrolytes. Membranes 10(11):363. https://doi.org/10.3390/membranes10110363
Soares BG, Leyva ME, Barra GM, Khastgir D (2006) Dielectric behavior of polyaniline synthesized by different techniques. Eur Polym J 42(3):676–686. https://doi.org/10.1016/j.eurpolymj.2005.08.013
Chérif SF, Cherif A, Dridi W, Zid MF (2020) AC conductivity, electric modulus analysis, dielectric behavior and bond valence sum analysis of Na3Nb4As3O19 compound. Arab J Chem 13(6):5627–5638. https://doi.org/10.1016/j.arabjc.2020.04.003
Ganta KK, Jeedi VR, Katrapally VK, Yalla M, Emmadi LN (2021) Effect of TiO2 nano-filler on electrical properties of Na+ ion conducting PEO/PVDF based blended polymer electrolyte. J Inorg Organomet Polym Mater 31:3430–3440. https://doi.org/10.1007/s10904-021-01947-w
Wang W, Alexandridis P (2016) Composite polymer electrolytes: nanoparticles affect structure and properties. Polymers 8(11):387. https://doi.org/10.3390/polym8110387
Bard AJ, Faulkner LR (2000) Electrochemical methods: fundamentals and applications, 2nd edn. Wiley, New York
Agrawal RC, Sahu DK, Mahipal YK, Ashrafi R (2013) Investigations on ion transport properties of hot-press cast magnesium ion conducting nano-composite polymer electrolyte (NCPE) films: effect of filler particle dispersal on room temperature conductivity. Mater Chem Phys 139:410–415. https://doi.org/10.1016/j.matchemphys.2012.12.056
Hadi JM, Aziz SB, Mustafa MS, Hamsan MH, Abdulwahid RT, Kadir MF, Ghareeb HO (2020) Role of nano-capacitor on dielectric constant enhancement in PEO: NH4SCN: xCeO2 polymer nano-composites: electrical and electrochemical properties. J Mater Res Technol 9(4):9283–9294. https://doi.org/10.1016/j.jmrt.2020.06.022
Hadi JM, Aziz SB, Nofal MM, Hussein SA, Hafiz MH, Brza MA, Abdulwahid RT, Kadir MF, Woo HJ (2020) Electrical, dielectric property and electrochemical performances of plasticized silver ion-conducting chitosan-based polymer nanocomposites. Membranes 10(7):151. https://doi.org/10.3390/membranes10070151
Dannoun EM, Aziz SB, Brza MA, Al-Saeedi SI, Nofal MM, Mishra K, Abdullah RM, Karim WO, Hadi JM (2022) Electrochemical and ion transport studies of Li+ ion-conducting MC-based biopolymer blend electrolytes. Int J Mol Sci 23(16):9152. https://doi.org/10.3390/ijms23169152
Yesappa L, Niranjana M, Ashokkumar SP, Vijeth H, Basappa M, Dwivedi J, Petwal VC, Ganesh S, Devendrappa H (2018) Optical properties and ionic conductivity studies of an 8 MeV electron beam irradiated poly (vinylidene fluoride-co-hexafluoropropylene)/LiClO4 electrolyte film for opto-electronic applications. RSC Adv 8:15297–15309. https://doi.org/10.1039/c8ra00970h
Bai J, Lu H, Cao Y, Li X, Wang J (2017) A novel ionic liquid polymer electrolyte for quasi-solid state lithium air batteries. RSC Adv 7(49):30603–30609. https://doi.org/10.1039/C7RA05035F
Hadi JM, Aziz SB, Kadir MF, El-Badry YA, Ahamad T, Hussein EE, Asnawi AS, Abdullah RM, Alshehri SM (2021) Design of plasticized proton conducting Chitosan: Dextran based biopolymer blend electrolytes for EDLC application: structural, impedance and electrochemical studies. Arab J Chem 14(11):103394. https://doi.org/10.1016/j.arabjc.2021.103394
Hüfner S (1995) Photoelectron spectroscopy: principles and applications. Springer, Berlin
Jeschull F, Maibach J, Edström K, Brandell D (2017) On the electrochemical properties and interphase composition of graphite: PVdF-HFP electrodes in dependence of binder content. J Electrochem Soc 164(7):A1765-1772. https://doi.org/10.1149/2.0121709jes
Gilbert JB, Luo M, Shelton CK, Rubner MF, Cohen RE, Epps TH III (2015) Determination of lithium-ion distributions in nanostructured block polymer electrolyte thin films by X-ray photoelectron spectroscopy depth profiling. ACS Nano 9(1):512–520. https://doi.org/10.1021/nn505744r
Xu C, Sun B, Gustafsson T, Edström K, Brandell D, Hahlin M (2014) Interface layer formation in solid polymer electrolyte lithium batteries: an XPS study. J Mater Chem A 2(20):7256–7264. https://doi.org/10.1039/C4TA00214H
Gregorio R Jr (2006) Determination of the α, β, and γ crystalline phases of poly (vinylidene fluoride) films prepared at different conditions. J Appl Polym Sci 100(4):3272–3279. https://doi.org/10.1002/app.23137
Vyas MK, Chandra A (2019) Synergistic effect of conducting and insulating fillers in polymer nanocomposite films for attenuation of X-band. J Mater Sci 54(2):1304–1325. https://doi.org/10.1007/s10853-018-2894-z
Parangusan H, Ponnamma D, AlMaadeed MA (2017) Flexible tri-layer piezoelectric nanogenerator based on PVDF-HFP/Ni-doped ZnO nanocomposites. RSC Adv 7(79):50156–50165. https://doi.org/10.1039/C7RA10223B
Al-Gharabli S, Al-Omari B, Kujawski W, Kujawa J (2020) Biomimetic hybrid membranes with covalently anchored chitosan—material design, transport and separation. Desalination 491:114550. https://doi.org/10.1016/j.desal.2020.114550
Qiao H, Wei Z, Yang H, Zhu L, Yan X (2009) Preparation and characterization of NiO nanoparticles by anodic arc plasma method. J Nanomater 2009:1–5. https://doi.org/10.1155/2009/795928
Lopes AC, Costa CM, Tavares CJ, Neves IC, Lanceros-Mendez S (2011) Nucleation of the electroactive γ phase and enhancement of the optical transparency in low filler content poly (vinylidene)/clay nanocomposites. J Phys Chem C 115(37):18076–18082. https://doi.org/10.1021/jp204513w
Oumghar K, Chakhchaoui N, Farhane R, Eddiai A, Meddad M, Cherkaoui O, Van Langenhove L (2020) Enhanced piezoelectric properties of PVdF-HFP/PZT nanocomposite for energy harvesting application. IOP Conf Ser 827:012034. https://doi.org/10.1088/1757-899X/827/1/012034
Huang W, Frech R, Wheeler RA (1994) Molecular structures and normal vibrations of trifluoromethane sulfonate (CF3SO3-) and its lithium ion pairs and aggregates. J Phys Chem 98(1):100–110. https://doi.org/10.1021/j100052a018
Fahmi EM, Ahmad A, Rahman MY, Hamzah H (2012) Effect of NiO nanofiller concentration on the properties of PEO-NiO-LiClO4 composite polymer electrolyte. J Solid State Electrochem 16:2487–2491. https://doi.org/10.1007/s10008-011-1638-7
Acknowledgements
We would like to extend gratitude to The Chairman, the Board of Studies, and the Head of the Department at the Department of Physics, Osmania University. Special thanks to Dr. K. Uday Kumar, Assistant Professor, Department of Physics, NIT, Warangal for providing Chronoamperometry and Cyclic Voltammetry investigations. The Principal of Government Polytechnic in Gadwal, Sri T. Ram Mohan, has provided Mr. P. Ajay Kumar solely with encouragement and support throughout his time at this work.
Funding
The authors have no funding available to disclose.
Author information
Authors and Affiliations
Contributions
PAK: conceptualization, methodology, investigation, validation, writing—original draft preparation, software, data curation, and editing. JSK: reviewing, editing, and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, P.A., Kumar, J.S. Environmentally Benign Nanofiller-Aided Enhancement of the Electrochemical Performance of Flexible Lithium Polymer Composite. J Polym Environ 32, 1631–1649 (2024). https://doi.org/10.1007/s10924-023-03064-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-023-03064-w