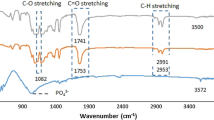
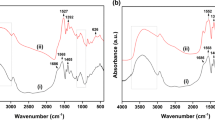
Abstract
In this study, a hydrogel adsorbent was successfully synthesized using hydroxyapatite/acrylamide/β-cyclodextrin to adsorb heavy metal ions and dyes in water. In the study, different contents of hydroxyapatite were added to the hydrogel, and the mechanical properties and adsorption properties of the material were studied. Results showed that the compressive strength of the hydrogel added with hydroxyapatite increased by 37.1%. The adsorption isotherm model demonstrated that the maximum adsorption capacity was 300 mg/g. In addition, the preparation process of the composite hydrogel was simple and could be used at a low pH for multiple times. Therefore, the material is of great significance to the treatment of pollutants.








Similar content being viewed by others
References
Macías-García A, Corzo MG, Domínguez MA, Franco MA, Naharro JM (2017) Study of the adsorption and electroadsorption process of Cu (II) ions within thermally and chemically modified activated carbon. J Hazard Mater 328:46–55
Zhu F, Li L, **ng J (2017) Selective adsorption behavior of Cd (II) ion imprinted polymers synthesized by microwave-assisted inverse emulsion polymerization: Adsorption performance and mechanism. J Hazard Mater 321:103–110
Al-Rashdi B, Johnson D, Hilal N (2013) Removal of heavy metal ions by nanofiltration. Desalination 315:2–17
Anitha K, Namsani S, Singh JK (2015) Removal of heavy metal ions using a functionalized single-walled carbon nanotube: a molecular dynamics study. The Journal of Physical Chemistry A 119(30):8349–8358
Gogoi N, Barooah M, Majumdar G, Chowdhury D (2015) Carbon dots rooted agarose hydrogel hybrid platform for optical detection and separation of heavy metal ions. ACS Appl Mater Interfaces 7(5):3058–3067
Xu B, Wang X, Huang Y, Liu J, Wang D, Feng S et al (2020) Electrospinning preparation of PAN/TiO2/PANI hybrid fiber membrane with highly selective adsorption and photocatalytic regeneration properties. Chem Eng J 399:125749
Xu R, Zhou G, Tang Y, Chu L, Liu C, Zeng Z et al (2015) New double network hydrogel adsorbent: Highly efficient removal of Cd (II) and Mn (II) ions in aqueous solution. Chem Eng J 275:179–188
Phetphaisit CW, Yuanyang S, Chaiyasith WC (2016) Polyacrylamido-2-methyl-1-propane sulfonic acid-grafted-natural rubber as bio-adsorbent for heavy metal removal from aqueous standard solution and industrial wastewater. J Hazard Mater 301:163–171
Repo E, Warchoł JK, Bhatnagar A, Mudhoo A, Sillanpää M (2013) Aminopolycarboxylic acid functionalized adsorbents for heavy metals removal from water. Water Res 47(14):4812–4832
Tan L, Wang S, Du W, Hu T (2016) Effect of water chemistries on adsorption of Cs (I) onto graphene oxide investigated by batch and modeling techniques. Chem Eng J 292:92–97
Echeverria C, Fernandes SN, Godinho MH, Borges JP, Soares PI (2018) Functional stimuli-responsive gels: Hydrogels and microgels. Gels 4(2):54
Liao H, Liu Y, Wang Q, Duan W (2019) Preparation and properties of a poly (vinyl alcohol) hydrogel-melamine formaldehyde foam composite. Polym Compos 40(5):2067–2075
Anjum S, Gurave P, Badiger MV, Torris A, Tiwari N, Gupta B (2017) Design and development of trivalent aluminum ions induced self-healing polyacrylic acid novel hydrogels. Polymer 126:196–205
Martín C, Merino S, González-Domínguez JM, Rauti R, Ballerini L, Prato M et al (2017) Graphene improves the biocompatibility of polyacrylamide hydrogels: 3D polymeric scaffolds for neuronal growth. Sci Rep 7(1):1–12
Chu L, Liu C, Zhou G, Xu R, Tang Y, Zeng Z et al (2015) A double network gel as low cost and easy recycle adsorbent: highly efficient removal of Cd (II) and Pb (II) pollutants from wastewater. J Hazard Mater 300:153–160
Ma J, Liu Y, Ali O, Wei Y, Zhang S, Zhang Y et al (2018) Fast adsorption of heavy metal ions by waste cotton fabrics based double network hydrogel and influencing factors insight. J Hazard Mater 344:1034–1042
Zhou G, Liu C, Chu L, Tang Y, Luo S (2016) Rapid and efficient treatment of wastewater with high-concentration heavy metals using a new type of hydrogel-based adsorption process. Bioresour Technol 219:451–457
Ma J, Jiang Z, Cao J, Yu F (2020) Enhanced adsorption for the removal of antibiotics by carbon nanotubes/graphene oxide/sodium alginate triple-network nanocomposite hydrogels in aqueous solutions. Chemosphere 242:125188
Wang Z, Li T-T, Peng H-K, Ren H-T, Lou C-W, Lin J-H (2021) Low-cost hydrogel adsorbent enhanced by trihydroxy melamine and β-cyclodextrin for the removal of Pb (II) and Ni (II) in water. J Hazard Mater 411:125029
Li T-T, **ng M, Gao B, Ren H-T, Peng H-K, Zhang X et al (2021) Multiscale synergistic toughened pluronic/PMEA/hydroxyapatite hydrogel laminated aramid soft composites: Puncture resistance and self-healing properties. Composites Part B: Engineering 216:108856
Li T-T, Zhang Y, Ren H-T, Peng H-K, Lou C-W, Lin J-H (2021) Two-step strategy for constructing hierarchical pore structured chitosan–hydroxyapatite composite scaffolds for bone tissue engineering. Carbohyd Polym 260:117765
Chen Y, Peng J, **ao H, Peng H, Bu L, Pan Z et al (2017) Adsorption behavior of hydrotalcite-like modified bentonite for Pb2+, Cu2+ and methyl orange removal from water. Appl Surf Sci 420:773–781
Liu X, Cheng C, **ao C, Shao D, Xu Z, Wang J et al (2017) Polyaniline (PANI) modified bentonite by plasma technique for U (VI) removal from aqueous solution. Appl Surf Sci 411:331–337
Nayak AK (2010) Hydroxyapatite synthesis methodologies: an overview. International Journal of ChemTech Research 2(2):903–907
Abollino O, Giacomino A, Malandrino M, Mentasti E (2008) Interaction of metal ions with montmorillonite and vermiculite. Appl Clay Sci 38(3-4):227–236
Sellaoui L, Soetaredjo FE, Ismadji S, Bonilla-Petriciolet A, Belver C, Bedia J et al (2018) Insights on the statistical physics modeling of the adsorption of Cd2+ and Pb2+ ions on bentonite-chitosan composite in single and binary systems. Chem Eng J 354:569–576
Nosrati H, Mamoory RS, Le DQS, Bünger CE (2020) Fabrication of gelatin/hydroxyapatite/3D-graphene scaffolds by a hydrogel 3D-printing method. Mater Chem Phys 239:122305
Sharma K, Sharma S, Sharma V, Mishra PK, Ekielski A, Sharma V et al (2021) Methylene Blue Dye Adsorption from Wastewater Using Hydroxyapatite/Gold Nanocomposite: Kinetic and Thermodynamics Studies. Nanomaterials 11(6):1403
Abd Jelil R (2015) A review of low-temperature plasma treatment of textile materials. Journal of Materials Science 50(18):5913–5943
Sun J, Li L, Kong Q, Zhang Y, Zhao P, Ge S et al (2019) Mimic peroxidase-transfer enhancement of photoelectrochemical aptasensing via CuO nanoflowers functionalized lab-on-paper device with a controllable fluid separator. Biosensors Bioelectronics 133:32–38
Zhao G, Li J, Ren X, Chen C, Wang X (2011) Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ Sci Technol 45(24):10454–10462
Daelemans L, Steyaert I, Schoolaert E, Goudenhooft C, Rahier H, De Clerck K (2018) Nanostructured hydrogels by blend electrospinning of polycaprolactone/gelatin nanofibers. Nanomaterials 8(7):551
Liu M, Chen C, Hu J, Wu X, Wang X (2011) Synthesis of magnetite/graphene oxide composite and application for cobalt (II) removal. J Phys Chem C 115(51):25234–25240
Cabaniss SE (2011) Forward modeling of metal complexation by NOM: II. Prediction of binding site properties. Environ Sci Technol 45(8):3202–3209
Jiang M-q, Wang Q-p, ** X-y, Chen Z-l (2009) Removal of Pb (II) from aqueous solution using modified and unmodified kaolinite clay. J Hazard Mater 170(1):332–339
Sari A, Tuzen M, Citak D, Soylak M (2007) Equilibrium, kinetic and thermodynamic studies of adsorption of Pb (II) from aqueous solution onto Turkish kaolinite clay. J Hazard Mater 149(2):283–291
Dou X, Mohan D, Pittman CU Jr (2013) Arsenate adsorption on three types of granular schwertmannite. Water Res 47(9):2938–2948
Vandenbossche M, Vezin H, Touati N, Jimenez M, Casetta M, Traisnel M (2014) Cysteine-grafted nonwoven geotextile: A new and efficient material for heavy metals sorption–Part B. Journal of environmental management 143:99–105
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156(1):2–10
Dada A, Olalekan A, Olatunya A, Dada O. Langmuir (2012) Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR Journal of Applied Chemistry 3(1):38–45
Liu Y, You CC, Li B (2001) Synthesis and molecular recognition of novel oligo (ethylenediamino) bridged bis (β-cyclodextrin) s and their copper (II) complexes: enhanced molecular binding ability and selectivity by multiple recognition. Chemistry–A European Journal 7(6):1281–1288
Bergaoui M, Nakhli A, Benguerba Y, Khalfaoui M, Erto A, Soetaredjo FE et al (2018) Novel insights into the adsorption mechanism of methylene blue onto organo-bentonite: Adsorption isotherms modeling and molecular simulation. J Mol Liq 272:697–707
Huang Z, Li Y, Chen W, Shi J, Zhang N, Wang X et al (2017) Modified bentonite adsorption of organic pollutants of dye wastewater. Mater Chem Phys 202:266–276
Pakdel PM, Peighambardoust SJ (2018) A review on acrylic based hydrogels and their applications in wastewater treatment. Journal of environmental management 217:123–143
Qi H-X, Zhai S-R, Wang Z-Z, Zhai B, An Q-D (2015) Designing recyclable Cu/ZrSBA-15 for efficient thiophene removal. Microporous Mesoporous Mater 217:21–29
Zendehdel M, Zamani F (2017) MCM-41-supported NNO type Schiff base complexes: a highly selective heterogeneous nanocatalyst for esterification, Diels–Alder and aldol condensation. J Porous Mater 24(5):1263–1277
Sun J, Cui K, Li L, Zhang L, Yu J (2020) Visible-light-driven renewable photoelectrochemical/synchronous visualized sensing platform based on Ni: FeOOH/BiVO4 photoanode and enzymatic cascade amplification for carcinoembryonic antigen detection. Sens Actuators B 304:127301
Ma W, Ding Y, Zhang M, Gao S, Li Y, Huang C et al (2020) Nature-inspired chemistry toward hierarchical superhydrophobic, antibacterial and biocompatible nanofibrous membranes for effective UV-shielding, self-cleaning and oil-water separation. J Hazard Mater 384:121476
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, ZK., Li, TT., Peng, HK. et al. Preparation and Adsorption Performance of Nano-hydroxyapatite-Enhanced Acrylamide Hydrogel Adsorbent. J Polym Environ 30, 2919–2927 (2022). https://doi.org/10.1007/s10924-021-02370-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02370-5