Abstract
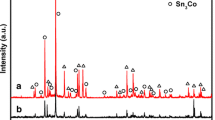
The pure semiconductor SnO2 hollow nanosphere (HNS) was synthesized by a carbon sphere (CS) template-assisted in solution phase growth method using Tin (IV) chloride (SnCl4). This HNS is fabricated or formed by aggregations among the adjacent SnO2 nanoparticles at above 100 °C temperature. The difference between the valance and conduction bands gap is found to be 3.387 eV. The whole diameter of the SnO2 HNS is ranged from 300–500 nm in size with the thickness of the wall 40–50 nm. The SnO2 nanoparticle size ranges from 20 to 30 nm. The dimension of the HNS are controlled by adjusting the some parameters, such as the concentration of SnCl4, the aging time, temperature, and the size of carbon spheres. The whole HNS size and wall thickness are affected by the Sn4+, due to the electrostatic force between CS and metal ions. The fabricated semiconductor SnO2 HNS was characterized by various analytical methods such as diffusion reflectance spectrum, Fourier transform infrared spectra, an X-ray diffraction pattern, Energy-dispersive X-ray pattern, Field emission scanning electron microscope (FESEM), and High-resolution transmittance electron microscope. Then, this semiconductor SnO2 hollow nanosphere-modified glassy carbon electrode exhibited excellent electrocatalytic activity for the reduction of hydrogen peroxide (H2O2) and ethanol with corresponding electro potential appeared at − 0.630 and − 0.624 V respectively. The oxidation of bioactive molecules such as Ascorbic acid, Dopamine, and Propyl gallate, with oxidation potential appeared at 0.634, 0.698, and 0.526 V respectively in low reduction potential.








Similar content being viewed by others
References
Z. Qingrui, G. Yang, B. Xue, W. Changzheng, X. Yi, Eur. J. Inorg. Chem. 2006, 1643–1648 (2006)
M.U. Khalid, S.R. Khan, S. Jamil, J. Inorg. Organomet. Polym. Mater. (2018). https://doi.org/10.1007/s10904-017-0687-5
I. Saadeddin, B. Pecquenard, J.P. Manaud, R. Decourt, C. Labruge`re, T. Buffeteau, G. Campet, Appl. Surf. Sci. 253, 5240–5249 (2007)
X.W. Lou, L.A. Archer, Z.C. Yang, Adv. Mater. 20, 3987 (2008)
M. Alexander, K. Pandian, J. Solid State Electrochem. 17, 1117–1125 (2013)
E. Ozkazanc, H. Ozkazanc, O. Gundogdu, J. Inorg. Organomet. Polym. Mater. (2018). https://doi.org/10.1007/s10904-018-0887-7
X.M. Sun, Y.D. Li, Angew. Chem. Int. Ed. 43, 3827 (2004)
K. Mohamed, J.A. Belen, F. Mc Millan Paul, E.A. Abil, C.P. Robert, L.J. Antonio, T.D. Maria, ACS Omega 3, 13227–13238 (2018)
M. Alexander, A. Saravanan, P. Arjun, K. Pandian, Anal. Sci. (2023). https://doi.org/10.1007/s44211-023-00317-5
N.A. Stephenson, A.T. Bell, Anal. Bioanal. Chem. 381, 1289 (2005)
S. Wei, W. **uzheng, W. Yuhua, J. **aomei, X. Li, L. Guangjiu, S. Zhenfan, Electrochim. Acta 87, 317–322 (2013)
M.M. Rahman, W.A. Adeosun, A.M. Asiri, Microchem. J. 159, 105536 (2020)
F.A. Harraz, A.A. Ismail, A.A. Ibrahim, S.A. Al-Sayari, M.S. Al-Assiri, Chem. Phys. Lett. 639, 238–242 (2015)
M. Alexander, K. Pandian, J Solid State Electrochem. 17, 2173–2182 (2013)
M.M. Hussain, A.M. Asiri, M.M. Rahman, Microchem. J. 159, 105534 (2020)
M. Alexander, S. Suriyadharshini, S. Raghu, Ra. Kalaivani, S. Gnanam, Mater. Sci. Semicond. Process. 99, 62–67 (2019)
C. Nayral, E. Viala, P. Fau, F. Senocq, C. Jumas, A. Maisonnat, B. Chaudret, Chem. Eur. J. 6, 4082 (2000)
M.M. Rahman, A.M. Asiri, RSC Adv. 5, 63252–63263 (2015)
M. Parthibavarman, V. Hariharan, C. Sekar, V.N. Singh, J. Optoelectro. Adv. Mater. 12, 1894 (2010)
M.R. Arefi-Rad, H. Kafashan, Opt. Mater. 105, 109887 (2020)
A. Alasvand, H. Kafashan, J. Alloy. Compd. 817, 152711 (2020)
W.W. Wang, Y.J. Zhu, L.X. Yang, Adv. Funct. Mater. 17, 59 (2007)
H.A. **, C.H. Chul, P.K. Won, K.S. Bin, J. Phys. Chem. B 108, 9815 (2004)
M. Li, Q. Wu, M. Wen, J. Shi, Nanoscale Res. Lett. 4, 1365 (2009)
S. Gnanam, V. Rajendran, J. Sol.-Gel. Sci. Technol. 53, 555–559 (2010)
N. Jia, Q. Zhou, L. Liu, M. Yan, Z. Jiang, J. Electroanal. Chem. 580, 213 (2005)
H.R. Kim, K.I. Choi, K.M. Kim, I.D. Kim, Chem. Commun. 46, 5061 (2010)
F. Li, J. Xu, X. Yu, L. Chen, J. Zhu, Z. Yang, Sensor and Actuator B 81, 165 (2002)
D.L. McCorkle, R.J. Warmack, S.V. Patel, T. Mlsna, S.R. Hunter, T.L. Ferrell, Sens. Actuators B 107, 892 (2005)
F.A. Harraz, A.A. Ismail, H. Bouzid, S.A. Al-Sayari, A. Al-Hajry, M.S. Al-Assiri, Appl. Sur. Sci. 307, 704 (2014)
M.M. Alam, A.M. Asiri, M.M.M.A. RahmanIslam, SN Appl. Sci. 2, 1953 (2020)
C. Haijie, L. Haiquan, N. Tianjun, P. Yingjie, Z. Yong, Z. Yongheng, Front. Chem. 7, 843 (2019)
S. Ling, W. Na, L. Wei, Y. Guangming, W. Zefeng, Int. J. Electrochem. Sci. (2021). https://doi.org/10.20964/2021.05.27
M. **, C. Meifeng, L. Hua, X. Shouqing, Z. Qi, W. Xueliang, Microchem. J. 183, 107954 (2022)
Acknowledgements
The authors extend their appreciation to researchers supporting project number (RSP2023R165), King Saud University Riyadh, Saudi Arabia.
Professor. Dr. K Pandian, Department of Inorganic Chemistry, University of Madras, Guindy Campus, Chennai- 600025, Tamil Nadu, India.
Author information
Authors and Affiliations
Contributions
AM did all the research work and wrote the main manuscript text. RP gives lab support. DA contributed to taking FESEM images. VRMR took HRTEM images.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marimuthu, A., Periyannan, R., Ali, D. et al. Synthesis of Semiconductor SnO2 Hollow Nanosphere; Their Modified Electrode for Electrochemical Reduction and Determination of Hydrogen Peroxide, Ethanol and Oxidation of Bioactive Molecules. J Inorg Organomet Polym 33, 2943–2953 (2023). https://doi.org/10.1007/s10904-023-02734-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-023-02734-5