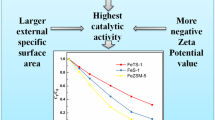
Abstract
In this paper, phenol was used as the material to be treated, and HMS molecular sieve was used as the catalyst for the catalytic treatment of the material. HMS, Ti-HMS, Ce-HMS and composite modified Ce/Ti-HMS molecules were used to prepare sieved catalysts. Simulated phenol-containing wastewater with phenol as the main component is treated. The structure of the molecular sieve and its elements and contents were characterized by field emission scanning electron microscope; the morphology of atoms or molecules in the material was characterized by X-ray diffractometer; the chemical bonds in the molecular structure were characterized by Fourier transform infrared spectroscopy. The change of vibration peak was analyzed, and the change of surface and skeleton structure was analyzed; the absorbance of the substance to be tested to visible light was measured by ultraviolet–visible diffuse reflectance method. The experiment was optimized by response surface methodology, and the oxidation of Ce3+ was detected by infrared tracking, and the reaction mechanism of phenol catalytic degradation was analyzed. The active sites that determine the catalytic performance of the composite catalyst were studied by DFT calculations, which further confirmed that the bimetallic modified zeolite has better catalytic effect.








Similar content being viewed by others
References
X. Li, Z. Lin, Q. Yuan et al., A highly effective and reusable platinum nanoblock based on graphene/polyamino acid nanofilms for 4-nitrophenol degradation. Appl. Surf. Sci. 589, 153029 (2022)
P.B. Patil, V.M. Bhandari, Solvent-assisted cavitation for enhanced removal of organic pollutants-degradation of 4-aminophenol. J. Environ. Manag. 311, 114857 (2022)
L. Hu, X. Liu, A. Guo et al., Cobalt with porous carbon architecture: towards of 4-nitrophenol degradation and reduction. Sep. Purif. Technol. 288, 120595 (2022)
F. Yuting, L. Changbo, Z. Guozheng, L. Hui, W. Shuo, X. Hongzhu, Progress in treatment technology of phenol-containing industrial wastewater. IOP Conf. Ser. Earth Environ. Sci. 787(1), 012054 (2021)
S. Ali, M. Humayun, W. Pi et al., Fabrication of BiFeO3-g-C3N4-WO3 Z-scheme heterojunction as highly efficient visible-light photocatalyst for water reduction and 2, 4-dichlorophenol degradation: insight mechanism. J. Hazard. Mater. 397, 122708 (2020)
L. Yin, J. Wei, Y. Qi et al., Degradation of pentachlorophenol in peroxymonosulfate/heat system: kinetics, mechanism, and theoretical calculations. Chem. Eng. J. 434, 134736 (2022)
P. Sarkar, S. De, S. Neogi, Microwave assisted facile fabrication of dual Z-scheme g-C3N4/ZnFe2O4/Bi2S3 photocatalyst for peroxymonosulphate mediated degradation of 2, 4, 6-trichlorophenol: the mechanistic insights. Appl. Catal. B Environ. 307, 121165 (2022)
T.T. Nazos, D.F. Ghanotakis, Biodegradation of phenol by alginate immobilized Chlamydomonas reinhardtii cells. Arch. Microbiol. 203(9), 5805–5816 (2021)
Y. Yuan, R. Guo, L. Hong et al., Fabrication of a dual S-scheme Bi7O9I3/g-C3N4/Bi3O4Cl heterojunction with enhanced visible-light-driven performance for phenol degradation. Chemosphere 287, 132241 (2022)
A.E. Oluwalana, P.A. Ajibade, Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol. Nanotechnol. Rev. 11(1), 883–896 (2022)
X. Liu, J. Zhou, D. Liu et al., Construction of Z-scheme CuFe2O4/MnO2 photocatalyst and activating peroxymonosulfate for phenol degradation: synergistic effect, degradation pathways, and mechanism. Environ. Res. 200, 111736 (2021)
C. Liu et al., Electrochemical treatment of phenol-containing wastewater by facet-tailored TiO2: efficiency, characteristics and mechanisms. Water Res. 165, 114980 (2019)
H. You, Z. Chen, Q. Yu et al., Preparation of a three-dimensional porous PbO2-CNTs composite electrode and study of the degradation behavior of p-nitrophenol. Sep. Purif. Technol. 276, 119406 (2021)
X. Li, X. Min, X. Hu et al., In-situ synthesis of highly dispersed Cu-CuxO nanoparticles on porous carbon for the enhanced persulfate activation for phenol degradation. Sep. Purif. Technol. 276, 119260 (2021)
J. Hu, P. Zhang, J. Cui, W. An, L. Liu, Y. Liang, Q. Yang, H. Yang, W. Cui, High-efficiency removal of phenol and coking wastewater via photocatalysis-Fenton synergy over a Fe-g-C3N4 graphene hydrogel 3D structure. J. Ind. Eng. Chem. 84, 305–314 (2020)
S. Feng, P. Zhang, W. Duan et al., P-nitrophenol degradation by pine-wood derived biochar: the role of redox-active moieties and pore structures. Sci. Total Environ. 741, 140431 (2020)
J. Chen, J. Wan, Y. Gong et al., Effective electro-Fenton-like process for phenol degradation on cerium oxide hollow spheres encapsulated in porous carbon cathode derived from skimmed cotton. Chemosphere 270, 128661 (2021)
M. Alegría, J. Aliaga, L. Ballesteros et al., Layered nanocomposite 2D-TiO2 with Cu2O nanoparticles as an efficient photocatalyst for 4-Chlorophenol degradation and hydrogen evolution. Top. Catal. 64(1), 167–180 (2021)
F. Liu, H. Zhang, Y. Yan, Stability and deactivation of graphene catalyst for phenol degradation in a fixed-bed reactor. Sep. Purif. Technol. 241, 116717 (2020)
M.N. Timofeeva, Z. Hasan, A.Y. Orlov et al., Fe-containing nickel phosphate zeolites as heterogeneous catalysts for phenol oxidation and hydroxylation with H2O2. Appl. Catal. B: Environ. 107(1), 197–204 (2011)
F. Ferri, L. Bertin, A. Scoma et al., Recovery of low molecular weight phenols through solid-phase extraction. Chem. Eng. J. 166(3), 994–1001 (2011)
A.E. Beck, Metabolic efficiency of sugar co-metabolism and phenol degradation in Alicyclobacillus acidocaldarius for improved lignocellulose processing. Processes 8(5), 502 (2020)
R.J. Wood, C. Vévert, J. Lee et al., Flow effects on phenol degradation and sonoluminescence at different ultrasonic frequencies. Ultrason. Sonochem. 63, 104892 (2020)
A. Kumar, P. Raizada, P. Singh et al., Facile synthesis and extended visible light activity of oxygen and sulphur co-doped carbon nitride quantum dots modified Bi2MoO6 for phenol degradation. J. Photochem. Photobiol. A Chem. 397, 112588 (2020)
P.J. Mafa, B.B. Mamba, A.T. Kuvarega, Photoelectrocatalytic evaluation of EG-CeO2 photoanode on degradation of 2, 4-dichlorophenol. Sol. Energy Mater. Sol. Cells 208, 110416 (2020)
D. Chen, A.K. Ray, Photocatalytic kinetics of phenol and its derivatives over UV irradiated TiO2. Appl. Catal. B Environ. 23(2–3), 143–157 (1999)
C. Adán, A. Bahamonde, M. Fernández-García et al., Structure and activity of nanosized iron-doped anatase TiO2 catalysts for phenol photocatalytic degradation. Appl. Catal. B Environ. 72(1–2), 11–17 (2007)
X. Qing, L. Yuan, Y. Wang et al., Synergistic influence of Cr3+ and CrO42− on the visible near-infrared spectrum of Mg-Al layered double hydroxides for efficient visible-light photocatalysis. J. Alloys Compds. 872, 159628 (2021)
N. Morales-Flores, U. Pal, E.S. Mora, Photocatalytic behavior of ZnO and Pt-incorporated ZnO nanoparticles in phenol degradation. Appl. Catal. A Gen. 394(1–2), 269–275 (2011)
N. Kashif, F. Ouyang, Parameters effect on heterogeneous photocatalysed degradation of phenol in aqueous dispersion of TiO2. J. Environ. Sci. 21(4), 527–533 (2009)
M.A. Barakat, J.M. Tseng, C.P. Huang, Hydrogen peroxide-assisted photocatalytic oxidation of phenolic compounds. Appl. Catal. B Environ. 59(1–2), 99–104 (2005)
C.H. Chiou, C.Y. Wu, R.S. Juang, Influence of operating parameters on photocatalytic degradation of phenol in UV/TiO2 process. Chem. Eng. J. 139(2), 322–329 (2008)
C.H. Chiou, R.S. Juang, Photocatalytic degradation of phenol in aqueous solutions by Pr-doped TiO2 nanoparticles. J. Hazard. Mater. 149(1), 1–7 (2007)
R.W. Matthews, S.R. McEvoy, Destruction of phenol in water with sun, sand, and photocatalysis. Sol. Energy 49(6), 507–513 (1992)
M. Falk, A.G. Miller, Infrared spectrum of carbon dioxide in aqueous solution. Vib. Spectrosc. 4(1), 105–108 (1992)
C. Tai, X. Gu, H. Zou et al., A new simple and sensitive fluorometric method for the determination of hydroxyl radical and its application. Talanta 58(4), 661–667 (2002)
A. Dobosz, A. Sobczyński, The influence of silver additives on titania photoactivity in the photooxidation of phenol. Water Res. 37(7), 1489–1496 (2003)
J. Al-Sabahi, T. Bora, M. Al-Abri et al., Controlled defects of zinc oxide nanorods for efficient visible light photocatalytic degradation of phenol. Materials 9(4), 238 (2016)
K. Bubacz, J. Choina, D. Dolat et al., Methylene blue and phenol photocatalytic degradation on nanoparticles of anatase TiO2. Pol. J. Environ. Stud. 19(4), 685–691 (2010)
J. Arana, E.P. Melian, V.M.R. López et al., Photocatalytic degradation of phenol and phenolic compounds: Part I. Adsorption and FTIR study. J. Hazard. Mater. 146(3), 520–528 (2007)
H. Wang, X. Chen, Kinetic analysis and energy efficiency of phenol degradation in a plasma-photocatalysis system. J. Hazard. Mater. 186(2–3), 1888–1892 (2011)
V. Brezová, A. Blažková, E. Borošová et al., The influence of dissolved metal ions on the photocatalytic degradation of phenol in aqueous TiO2 suspensions. J. Mol. Catal. A Chem. 98(2), 109–116 (1995)
K. Hayat, M.A. Gondal, M.M. Khaled et al., Effect of operational key parameters on photocatalytic degradation of phenol using nano nickel oxide synthesized by sol–gel method. J. Mol. Catal. A Chem. 336(1–2), 64–71 (2011)
Acknowledgements
We thank Science and Technology Program of Shaanxi Province (2020QFY05-05), Joint Fund of the Yulin University and the Dalian National Laboratory for Clean Energy (Grant. YLU-DNL Fund 2021003), Yulin Science and Technology Bureau (CXY-2021-108-02) and Yulin High-tech Zone Science and Technology Bureau (CXY-2020-031 and CXY-2021-22).
Author information
Authors and Affiliations
Contributions
YP: Drafting the manuscript Validation Resources Data Curation LG: Project administration Funding acquisition. MX:acquisition of data Visualization YF: Data Curation Conception and design of study XS: Conception and design of study, analysis and interpretation of data Conceptualization Software
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pang, Y., Gao, L., **a, M. et al. Nanostructure of Bimetallic Modified HMS Zeolite and Its Catalytic Effect on Phenol Degradation. J Inorg Organomet Polym 32, 3407–3416 (2022). https://doi.org/10.1007/s10904-022-02460-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-022-02460-4