Abstract
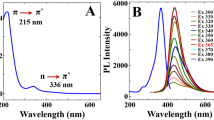
Bilirubin (BR), a heme protein produced from breakdown of haemoglobin is present in aged red blood cells; whose abnormal concentration is associated with diseases like hyperbilirubinemia, coronary disease, iron deficiency, and so on. Herein, we have synthesized a selective, sensitive, and low-cost sensing platform using fluorescent nitrogen doped carbon dots (NCDs), prepared from precursors; citric acid and urea via a simple microwave-assisted method. The emission at 444 nm on excitation with 360 nm was well quenched in presence of BR suggesting a direct turn-off detection for BR. Characterization of developed probe was done by UV-Visible absorption studies, photoluminescence studies, SEM, TEM, ATR-FTIR, XPS, and DLS analysis. BR was detected with a Limit of Detection (LoD) and Limit of Quantification (LoQ) of 0.32 µM and 1.08 µM respectively. NCDs exhibited excellent selectivity and sensitivity towards BR in the presence of co-existing biomolecules and ions. Practical feasibility was checked by paper-strip-based sensing of BR and spiked real human samples were used for conducting real sample analysis.






Similar content being viewed by others
Data Availability
Data made available on reasonable request.
References
Tenhunen R, Marver HS, Schmid R, Oxygenase MH (1969) Characterization of the enzyme. J Biol Chem 244:6388–6394. https://doi.org/10.1016/S0021-9258(18)63477-5
Hayaishi O, Nozaki M (1979) Nature and mechanisms of oxygenases. Science 164(1969):389–396. https://doi.org/10.1126/SCIENCE.164.3878.389/ASSET/E4257877-7D4D-4DB9-B49C-F33061B603DC/ASSETS/SCIENCE.164.3878.389.FP.PNG
Ndabakuranye JP, Li S, Burchall G, Fox K, Piva T, Xu Z, Kavehei O, Prawer S, Ahnood A (2022) 70 years of bilirubin sensing: towards the point-of- care bilirubin monitoring in cirrhosis and hyperbilirubinemia. Sens Diagnostics 1:932–954. https://doi.org/10.1039/d2sd00033d
Adin CA (2021) Bilirubin as a Therapeutic Molecule: Challenges and Opportunities. Antioxidants 10 (2021) 1536. https://doi.org/10.3390/ANTIOX10101536
Patra SK, Mandal AK, Pal MK (1999) State of aggregation of bilirubin in aqueous solution: principal component analysis approach. J Photochem Photobiol Chem 122:23–31. https://doi.org/10.1016/S1010-6030(98)00465-1
Vitek L, Ostrow J (2009) Bilirubin chemistry and metabolism; harmful and protective aspects. Curr Pharm Des 15:2869–2883. https://doi.org/10.2174/138161209789058237
BONNETT R, DAVIES JE, HURSTHOUSE MB (1976) Structure of bilirubin. Nature 262:326–328. https://doi.org/10.1038/262326a0
Aparna RS, Anjali AD, John N, Abha K, Syamchand SS, George S (2018) Blue emitting copper nanoclusters as colorimetric and fluorescent probe for the selective detection of bilirubin. Spectrochim Acta Mol Biomol Spectrosc 199:123–129. https://doi.org/10.1016/j.saa.2018.03.045
Schmid R, Jaundice, Metabolism B (1958) AMA Arch Intern Med 101:669–674. https://doi.org/10.1001/ARCHINTE.1958.00260150157022
Billing BH, Lathe GH (1958) Bilirubin metabolism in jaundice. Am J Med 24:111–121. https://doi.org/10.1016/0002-9343(58)90366-8
Arul P, Huang ST, Mani V (2021) Surfactant-induced morphological evolution of Cu(II) metal organic frameworks: Applicable in picomolar quantification of bilirubin. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2021.149827
Brodersen R, Stern L (1980) Binding of bilirubin to albumin. Crit Rev Clin Lab Sci 11:307–399. https://doi.org/10.3109/10408368009105860/ASSET//CMS/ASSET/DDC6B1EF-8957-47FD-B784-2DD872A88434/10408368009105860.FP.PNG
Yi K, Li H, Zhang X, Zhang L (2021) Designed tb(III)-Functionalized MOF-808 as visible fluorescent probes for monitoring bilirubin and identifying fingerprints. Inorg Chem 60:3172–3180. https://doi.org/10.1021/acs.inorgchem.0c03312
Bernhard K, Ritzel G, Steiner KU (1954) Über Eine Biologische Bedeutung Der Gallenfarbstoffe. Bilirubin Und Biliverdin als Antioxydantien für das vitamin A und die essentiellen Fettsäuren. Helv Chim Acta 37:306–313. https://doi.org/10.1002/HLCA.19540370139
Woodgate P, nthony LA, Jardine (2011) Neonatal jaundice, BMJ Clin Evid /pmc/articles/PMC3217664 / (accessed December 6, 2023)
Itoh S, Okada H, Koyano K, Nakamura S, Konishi Y, Iwase T, Kusaka T (2023) Fetal and neonatal bilirubin metabolism. Front Pediatr 10:1002408. https://doi.org/10.3389/FPED.2022.1002408/BIBTEX
Ngashangva L, Bachu V, Goswami P (2019) Development of new methods for determination of bilirubin. J Pharm Biomed Anal 162:272–285. https://doi.org/10.1016/j.jpba.2018.09.034
Skandalakis JE, Johnson RH, Rand EO (1954) Chronic idiopathic jaundice with unidentified pigment in liver cells; a new clinicopathologic entity with a report of 12 cases. Medicine 33:392–393. https://doi.org/10.1097/00005792-195409000-00001
Abha K, Nebu J, Anjali Devi JS, Aparna RS, Anjana RR, Aswathy AO, George S (2019) Photoluminescence sensing of bilirubin in human serum using L-cysteine tailored manganese doped zinc sulphide quantum dots. Sens Actuators B Chem 282:300–308. https://doi.org/10.1016/j.snb.2018.11.063
Crigler JF, Najjar VA (1952) Congenital Familial Nonhemolytic Jaundice with. Kernicterus Pediatrics 10:169–180. https://doi.org/10.1542/PEDS.10.2.169
Jayasree M, Aparna RS, Anjana RR, Anjali Devi JS, John N, Abha K, Manikandan A, George S (2018) Fluorescence turn on detection of bilirubin using Fe (III) modulated BSA stabilized copper nanocluster; a mechanistic perception. Anal Chim Acta 1031:152–160. https://doi.org/10.1016/j.aca.2018.05.026
Schmid R (1959) Jaundice and Bilirubin Metabolism. Bull N Y Acad Med 35:755. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1806280/. Accessed 10 Dec 2023
**a C, Xu Y, Cao MM, Liu YP, **a JF, Jiang DY, Zhou GH, **e RJ, Zhang DF, Li HL (2020) A selective and sensitive fluorescent probe for bilirubin in human serum based on europium(III) post-functionalized zr(IV)-Based MOFs. Talanta. https://doi.org/10.1016/j.talanta.2020.120795
Zhang C, Bai W, Yang Z (2016) A novel photoelectrochemical sensor for bilirubin based on porous transparent TiO2 and molecularly imprinted polypyrrole. Electrochim Acta 187:451–456. https://doi.org/10.1016/j.electacta.2015.11.098
Guo Y, Wei C, Wang R, **ang Y (2023) Nanomaterials for fluorescent assay of bilirubin. Anal Biochem. https://doi.org/10.1016/j.ab.2023.115078
Shanmugaraj K, John SA (2019) Water-soluble MoS2 quantum dots as effective fluorescence probe for the determination of bilirubin in human fluids. Spectrochim Acta Mol Biomol Spectrosc 215:290–296. https://doi.org/10.1016/j.saa.2019.02.104
Anjana RR, Anjali Devi JS, Jayasree M, Aparna RS, Aswathy B, Praveen GL, Lekha GM, Sony G (2018) S, N-doped carbon dots as a fluorescent probe for bilirubin. Microchim Acta. https://doi.org/10.1007/s00604-017-2574-8
Basu S, Sahoo AK, Paul A, Chattopadhyay A (2016) Thumb imprint based detection of Hyperbilirubinemia using luminescent gold nanoclusters. Sci Rep. https://doi.org/10.1038/srep39005
Devi JSA, Aparna RS, Aswathy B, Nebu J, Aswathy AO, George S (2019) Understanding the citric acid–Urea Co–Directed Microwave assisted synthesis and Ferric Ion Modulation of fluorescent Nitrogen Doped Carbon dots: a turn on assay for ascorbic acid. ChemistrySelect 4:816–824. https://doi.org/10.1002/slct.201803726
Xu Q, Kuang T, Liu Y, Cai L, Peng X, Sreenivasan Sreeprasad T, Zhao P, Yu Z, Li N (2016) Heteroatom-doped carbon dots: synthesis, characterization, properties, photoluminescence mechanism and biological applications. J Mater Chem B 4:7204–7219. https://doi.org/10.1039/C6TB02131J
Dong Y, Cai J, You X, Chi Y (2015) Sensing applications of luminescent carbon based dots. Analyst 140:7468–7486. https://doi.org/10.1039/C5AN01487E
Pan L, Sun S, Zhang A, Jiang K, Zhang L, Dong C, Huang Q, Wu A, Lin H (2015) Truly fluorescent excitation-dependent Carbon dots and their applications in Multicolor Cellular Imaging and Multidimensional Sensing. Adv Mater 27:7782–7787. https://doi.org/10.1002/ADMA.201503821
O AA, George S (2023) Picric Acid Incorporated fluorescent Nitrogen Doped Carbon dot for Turn-On detection of glutathione. J Fluoresc 1–10. https://doi.org/10.1007/S10895-023-03541-4/TABLES/1
Mazurenko I, Monsalve K, Rouhana J, Parent P, Laffon C, Le Goff A, Szunerits S, Boukherroub R, Giudici-Orticoni MT, Mano N, Lojou E (2016) How the intricate interactions between carbon nanotubes and two bilirubin oxidases control direct and mediated O2 reduction. ACS Appl Mater Interfaces 8:23074–23085. https://doi.org/10.1021/acsami.6b07355
Pawar S, Togiti UK, Bhattacharya A, Nag A (2019) Functionalized Chitosan-Carbon dots: a fluorescent probe for detecting Trace amount of Water in Organic solvents. ACS Omega 4:11301–11311. https://doi.org/10.1021/acsomega.9b01208
Zhang F, Wang M, Zeng D, Zhang H, Li Y, Su X (2019) A molybdenum disulfide quantum dots-based ratiometric fluorescence strategy for sensitive detection of epinephrine and ascorbic acid. Anal Chim Acta 1089:123–130. https://doi.org/10.1016/j.aca.2019.09.005
Stachowska JD, Murphy A, Mellor C, Fernandes D, Gibbons EN, Krysmann MJ, Kelarakis A, Burgaz E, Moore J, Yeates SG (2021) A rich gallery of carbon dots based photoluminescent suspensions and powders derived by citric acid/urea. Sci Rep. https://doi.org/10.1038/s41598-021-89984-w
Chen D, Gao H, Chen X, Fang G, Yuan S, Yuan Y (2017) Excitation-Independent Dual-Color Carbon dots: Surface-State Controlling and solid-state lighting. ACS Photonics 4:2352–2358. https://doi.org/10.1021/acsphotonics.7b00675
Nixon MS, Aguado AS (2012) Feature extraction and image processing for computer vision. Feature Extr Image Process Comput Vis. https://doi.org/10.1016/C2011-0-06935-1
Nandi S, Biswas S (2019) A recyclable post-synthetically modified Al(iii) based metal-organic framework for fast and selective fluorogenic recognition of bilirubin in human biofluids. Dalton Trans 48:9266–9275. https://doi.org/10.1039/c9dt01180c
Karmakar S, Das TK, Kundu S, Maiti S, Saha A (2020) Physicochemical Understanding of Protein-Bound Quantum dot-based sensitive probing of Bilirubin: validation with real samples and implications of protein conformation in sensing. ACS Appl Bio Mater 3:8820–8829. https://doi.org/10.1021/acsabm.0c01165
Mathew MS, Philip A, Joseph K (2020) Wheat -Gluten-Directed Facile Synthesis of AgAuQC: probing Inner Filter effects and Electron Transfer for Bilirubin Detection. ChemistrySelect 5:9641–9653. https://doi.org/10.1002/slct.202000010
**ao ZY, Yin JH (2013) Spectroscopic studies on bilirubin aggregate at liquid/liquid interface. Anal Bioanal Chem 405:2723–2728. https://doi.org/10.1007/s00216-012-6698-8
Rajamanikandan R, Ilanchelian M (2019) Red emitting human serum albumin templated copper nanoclusters as effective candidates for highly specific biosensing of bilirubin. Mater Sci Engineering: C 98:1064–1072. https://doi.org/10.1016/J.MSEC.2019.01.048
Sasikumar K, Rajamanikandan R, Ju H (2023) Nitrogen- and sulfur-codoped strong green fluorescent Carbon dots for the highly specific quantification of Quercetin in Food samples. Materials 16:7686. https://doi.org/10.3390/MA16247686/S1
**ao W, **ong Y, Li Y, Chen Z, Li H (2023) Non-Enzymatically colorimetric bilirubin sensing based on the catalytic structure disruption of gold nanocages. Sensors 23:2969. https://doi.org/10.3390/S23062969
Sasikumar K, Rajamanikandan R, Ju H (2023) Fluorescent carbon dots for highly sensitive bilirubin sensing with excellent selectivity. J Science: Adv Mater Devices 8:100599. https://doi.org/10.1016/J.JSAMD.2023.100599
Yola ML, Göde C, Atar N (2017) Molecular imprinting polymer with polyoxometalate/carbon nitride nanotubes for electrochemical recognition of bilirubin. Electrochim Acta 246:135–140. https://doi.org/10.1016/j.electacta.2017.06.053
Yang W, **a J, Zhou G, Jiang D, Li Q (2018) Sensitive detection of free bilirubin in blood serum using β-diketone modified europium-doped yttrium oxide nanosheets as a luminescent sensor. RSC Adv 8:17854–17859. https://doi.org/10.1039/c8ra02817f
Ndabakuranye JP, Rajapaksa AE, Burchall G, Li S, Prawer S, Ahnood A (2022) A Novel Optical Assay System for Bilirubin Concentration Measurement in whole blood. IEEE Trans Biomed Eng 69:983–990. https://doi.org/10.1109/TBME.2021.3111150
Grohmanna K, Roser M, Rolinski B, Kadow I, Müller C, Goerlach-Graw A, Nauck M, Küster H (2006) Bilirubin measurement for neonates: comparison of 9 frequently used methods. Pediatrics 117:1174–1183. https://doi.org/10.1542/PEDS.2005-0590
Jain S, Pandey K, Lahoti A (2013) Pk. Rao, evaluation of skin and subcutaneous tissue thickness at insulin injection sites in Indian, insulin naïve, type-2 diabetic adult population. Indian J Endocrinol Metab 17:864. https://doi.org/10.4103/2230-8210.117249
McEwen M, Reynolds K (2014) Noninvasive detection of bilirubin in discrete vessels
Acknowledgements
The authors are immensely thankful to the Head, Department of Chemistry, School of Physical and Mathematical Sciences, University of Kerala, Kariavattom, Thiruvananthapuram for providing the laboratory and instrumental facilities. The authors acknowledge the CLIF, University of Kerala, Thiruvananthapuram and International Inter University Centre for Sensing and Imaging (IIUCSI), Department of Chemistry, University of Kerala, Kariavattom, Thiruvananthapuram. The authors also thankful to STIC, CUSAT, Kochi, India.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study. The author 1 Aswathy A. O. conceived the idea of the work. Design, material preparation, data collection and analysis were performed by Aswathy (A) O. (author 1) and Akhila (B) A. (author 2). Supervision was done by author 3 Dr. Sony George. The first draft of the manuscript was written by Aswathy A. O. (author 1). All the authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
The experiments were done following the Indian Council of Medical Research (ICMR) ethical standards and were approved by the University level Human Ethical Committee order no (ULECRIHS/UOK/2019/48, University of Kerala, Thiruvananthapuram). All the healthy volunteers who donated the blood samples were provided consent to participate and publish the result.
Competing Interests
The authors declare no competing interests.
Supporting Information
Additional supporting information can be found online in the supporting information section at the end of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
O, A.A., Akhila, B.A. & George, S. Fluorescent Nitrogen-doped Carbon Dots-based Turn-off Sensor for Bilirubin. J Fluoresc (2024). https://doi.org/10.1007/s10895-024-03771-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-024-03771-0