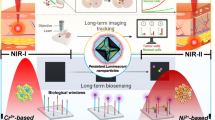
Abstract
Due to their persistent luminescence, persistent luminescent (PersL) materials have attracted great interest. In the biomedical field, the use of persistent luminescent nanoparticles (PLNPs) eliminates the need for continuous in situ excitation, thereby avoiding interference from tissue autofluorescence and significantly improving the signal-to-noise ratio (SNR). Although persistent luminescence materials can emit light continuously, the luminescence intensity of small-sized nanoparticles in vivo decays quickly. Early persistent luminescent nanoparticles were mostly excited by ultraviolet (UV) or visible light and were administered for imaging purposes through ex vivo charging followed by injection into the body. Limited by the low in vivo penetration depth, UV light cannot secondary charge PLNPs that have decayed in vivo, and visible light does not penetrate deep enough to reach deep tissues, which greatly limits the imaging time of persistent luminescent materials. In order to address this issue, the development of PLNPs that can be activated by light sources with superior tissue penetration capabilities is essential. Near-infrared (NIR) light and X-rays are widely recognized as ideal excitation sources, making persistent luminescent materials stimulated by these two sources a prominent area of research in recent years. This review describes NIR and X-ray excitable persistent luminescence materials and their recent advances in bioimaging.






Similar content being viewed by others
Data Availability
Not available.
References
Liu Y, Kuang J, Lei B, Shi C (2005) Color-control of long-lasting phosphorescence (LLP) through rare earth ion-doped cadmium metasilicate phosphors. J Mater Chem 15(37). https://doi.org/10.1039/b507774e
Wei X, Huang X, Zeng Y et al (2020) Longer and stronger: improving persistent luminescence in size-tuned zinc gallate nanoparticles by alcohol-mediated chromium do**. ACS Nano 14(9):12113–12124. https://doi.org/10.1021/acsnano.0c05655
Liu J, Liang Y, Yan S et al (2021) Sunlight-activated long persistent luminescence in the ultraviolet-B spectral region from Bi3+-doped garnet phosphors for covert optical tagging. J Mater Chem C 9(30):9692–9701. https://doi.org/10.1039/d1tc01922h
Hölsä J (2009) Persistent luminescence beats the afterglow: 400 years of persistent luminescence. Electrochem Soc Inte 18(4):42–45. https://doi.org/10.1149/2.F06094if
Matsuzawa T, Aoki Y, Takeuchi N, Murayama Y (2019) A new long phosphorescent phosphor with high brightness, SrAl2O4:Eu2+, Dy3+. J Electrochem Soc 143(8):2670–2673. https://doi.org/10.1149/1.1837067
Shi C, Fu Y, Liu B et al (2007) The roles of Eu2+ and Dy3+ in the blue long-lasting phosphor Sr2MgSi2O7:Eu2+,Dy3+. J Lumin 122–123:11–13. https://doi.org/10.1016/j.jlumin.2006.01.066
Lin L, Shi C, Wang Z, Zhang W, Yin M (2008) A kinetics model of red long-lasting phosphorescence in MgSiO3:Eu2+, Dy3+, Mn2+. J Alloy Compd 466(1–2):546–550. https://doi.org/10.1016/j.jallcom.2007.11.093
Aitasalo T, Hölsä J, Kirm M et al (2007) Persistent luminescence and synchrotron radiation study of the Ca2MgSi2O7:Eu2+,R3+ materials. Radiat Meas 42(4–5):644–647. https://doi.org/10.1016/j.radmeas.2007.01.058
Dorenbos P (2005) Mechanism of persistent luminescence in Eu2+ and Dy3+ codoped aluminate and silicate compounds. J Electrochem Soc 152(7). https://doi.org/10.1149/1.1926652
le Masne de Chermont Q, Chaneac C, Seguin J et al (2007) Nanoprobes with near-infrared persistent luminescence for in vivo imaging. Proc Natl Acad Sci U S A 104(22):9266–9271. https://doi.org/10.1073/pnas.0702427104
Aitasalo T, Holsa J, Jungner H, Lastusaari M, Niittykoski J (2006) Thermoluminescence study of persistent luminescence materials: Eu2+- and R3+-doped calcium aluminates, CaAl2O4:Eu2+,R3+. J Phys Chem B 110(10):4589–4598. https://doi.org/10.1021/jp057185m
Das S, Sharma SK, Manam J (2022) Near infrared emitting Cr3+ doped Zn3Ga2Ge2O10 long persistent phosphor for night vision surveillance and anti-counterfeit applications. Ceram Int 48(1):824–831. https://doi.org/10.1016/j.ceramint.2021.09.163
Zhu K, Chen Z, Wang Y et al (2022) (M,ca)AlSiN3:Eu2+ (M = sr, mg) long persistent phosphors prepared by combustion synthesis and applications in displays and optical information storage. J Lumin 252. https://doi.org/10.1016/j.jlumin.2022.119288
Chen LJ, Sun SK, Wang Y et al (2016) Activatable multifunctional persistent luminescence nanoparticle/copper sulfide nanoprobe for in vivo luminescence imaging-guided photothermal therapy. ACS Appl Mater Interfaces 8(48):32667–32674. https://doi.org/10.1021/acsami.6b10702
Fan W, Lu N, Xu C et al (2017) Enhanced afterglow performance of persistent luminescence implants for efficient repeatable photodynamic therapy. ACS Nano 11(6):5864–5872. https://doi.org/10.1021/acsnano.7b01505
Yang L, Gai S, Ding H et al (2023) Recent progress in inorganic afterglow materials: mechanisms, persistent luminescent properties, modulating methods, and bioimaging applications. Adv Opt Mater. https://doi.org/10.1002/adom.202202382
Maldiney T, Scherman D, Richard C (2012) Persistent luminescence nanoparticles for diagnostics and imaging. Functional Nanoparticles for Bioanalysis, Nanomedicine, and Bioelectronic Devices Volume 2. ACS Symposium Series, vol 1113: American Chemical Society; p. 1–25
Teston E, Maldiney T, Marangon I et al (2018) Nanohybrids with magnetic and persistent luminescence properties for cell labeling, tracking, in vivo real-time imaging, and magnetic vectorization. Small 14(16):e1800020. https://doi.org/10.1002/smll.201800020
Lecuyer T, Teston E, Ramirez-Garcia G et al (2016) Chemically engineered persistent luminescence nanoprobes for bioimaging. Theranostics 6(13):2488–2524. https://doi.org/10.7150/thno.16589
Liu J, Lecuyer T, Seguin J et al (2019) Imaging and therapeutic applications of persistent luminescence nanomaterials. Adv Drug Deliv Rev 138:193–210. https://doi.org/10.1016/j.addr.2018.10.015
Viana B, Richard C, Castaing V et al (2020) NIR-persistent luminescence nanoparticles for bioimaging, principle and perspectives. In: Benayas A, Hemmer E, Hong G, Jaque D (eds) Near infrared-emitting nanoparticles for biomedical applications. Springer International Publishing, Cham, pp 163–197
Cai G, Delgado T, Richard C, Viana B (2023) ZGSO spinel nanoparticles with dual emission of Nir persistent luminescence for anti-counterfeiting applications. Mater (Basel) 16(3). https://doi.org/10.3390/ma16031132
Cai G, Delgado T, Richard C, Viana B (2023) Transition metal and rare earth doped Zn1.3Ga1.4Sn0.3O4 persistent phosphors for anti-counterfeiting applications. SPIE OPTO. SPIE. https://doi.org/10.1117/12.2649803
Schnermann MJ (2017) Chemical biology: Organic dyes for deep bioimaging. Nature 551(7679):176–177. https://doi.org/10.1038/nature24755
Li B, Zhao M, Zhang F (2020) Rational design of near-infrared-II organic molecular dyes for bioimaging and biosensing. ACS Mater Lett 2(8):905–917. https://doi.org/10.1021/acsmaterialslett.0c00157
Zhu S, Tian R, Antaris AL, Chen X, Dai H (2019) Near-infrared-II molecular dyes for cancer imaging and Surgery. Adv Mater 31(24):e1900321. https://doi.org/10.1002/adma.201900321
Zhang Y, Song N, Li Y et al (2019) Comparative study of two near-infrared coumarin-BODIPY dyes for bioimaging and photothermal therapy of cancer. J Mater Chem B 7(30):4717–4724. https://doi.org/10.1039/c9tb01165j
Lei Z, Sun C, Pei P et al (2019) Stable, wavelength-tunable fluorescent dyes in the nir-ii region for in vivo high-contrast bioimaging and multiplexed biosensing. Angew Chem Int Ed Engl 58(24):8166–8171. https://doi.org/10.1002/anie.201904182
Zhou J, Yang Y, Zhang CY (2015) Toward biocompatible semiconductor quantum dots: from biosynthesis and bioconjugation to biomedical application. Chem Rev 115(21):11669–11717. https://doi.org/10.1021/acs.chemrev.5b00049
Ma JJ, Yu MX, Zhang Z et al (2018) Gd-DTPA-coupled Ag2Se quantum dots for dual-modality magnetic resonance imaging and fluorescence imaging in the second near-infrared window. Nanoscale 10(22):10699–10704. https://doi.org/10.1039/c8nr02017e
Ge XL, Huang B, Zhang ZL et al (2019) Glucose-functionalized near-infrared Ag2Se quantum dots with renal excretion ability for long-term in vivo Tumor imaging. J Mater Chem B 7(38):5782–5788. https://doi.org/10.1039/c9tb01112a
Morselli G, Villa M, Fermi A, Critchley K, Ceroni P (2021) Luminescent copper indium sulfide (CIS) quantum dots for bioimaging applications. Nanoscale Horiz 6(9):676–695. https://doi.org/10.1039/d1nh00260k
Arshad A, Akram R, Iqbal S et al (2019) Aqueous synthesis of tunable fluorescent, semiconductor CuInS2 quantum dots for bioimaging. Arab J Chem 12(8):4840–4847. https://doi.org/10.1016/j.arabjc.2016.10.002
Bai Y, Wang Y, Cao L et al (2021) Self-Targeting carbon quantum dots for peroxynitrite detection and imaging in live cells. Anal Chem 93(49):16466–16473. https://doi.org/10.1021/acs.analchem.1c03515
Kumar VB, Sher I, Rencus-Lazar S, Rotenstreich Y, Gazit E (2023) Functional carbon quantum dots for ocular imaging and therapeutic applications. Small 19(7):e2205754. https://doi.org/10.1002/smll.202205754
Zhao N, Wang Y, Hou S, Zhao L (2020) Functionalized carbon quantum dots as fluorescent nanoprobe for determination of tetracyclines and cell imaging. Mikrochim Acta 187(6):351. https://doi.org/10.1007/s00604-020-04328-1
Lu H, Li W, Dong H, Wei M (2019) Graphene quantum dots for optical bioimaging. Small 15(36):e1902136. https://doi.org/10.1002/smll.201902136
Campbell E, Hasan MT, Gonzalez-Rodriguez R et al (2021) Graphene quantum dot formulation for cancer imaging and redox-based drug delivery. Nanomedicine 37:102408. https://doi.org/10.1016/j.nano.2021.102408
Milenkovic M, Misovic A, Jovanovic D et al (2021) Facile synthesis of l-cysteine functionalized graphene quantum dots as a bioimaging and photosensitive agent. Nanomaterials (Basel) 11(8). https://doi.org/10.3390/nano11081879
He S, Chen S, Li D et al (2019) High affinity to skeleton rare earth doped nanoparticles for near-infrared II imaging. Nano Lett 19(5):2985–2992. https://doi.org/10.1021/acs.nanolett.9b00140
Zhao Z, Yuan J, Zhao X et al (2019) Engineering the infrared luminescence and photothermal properties of double-shelled rare-earth-doped nanoparticles for biomedical applications. ACS Biomater Sci Eng 5(8):4089–4101. https://doi.org/10.1021/acsbiomaterials.9b00526
Qu Z, Shen J, Li Q et al (2020) Near-IR emissive rare-earth nanoparticles for guided Surgery. Theranostics 10(6):2631–2644. https://doi.org/10.7150/thno.40808
Yu Z, Eich C, Cruz LJ (2020) Recent advances in rare-earth-doped nanoparticles for nir-ii imaging and cancer theranostics. Front Chem 8:496. https://doi.org/10.3389/fchem.2020.00496
Smith AM, Mancini MC, Nie S (2009) Bioimaging: second window for in vivo imaging. Nat Nanotechnol 4(11):710–711. https://doi.org/10.1038/nnano.2009.326
Ma Y, Chen Q, Pan X, Zhang J (2021) Insight into fluorescence imaging and bioorthogonal reactions in biological analysis. Top Curr Chem (Cham) 379(2):10. https://doi.org/10.1007/s41061-020-00323-5
Wu S, Li Y, Ding W et al (2020) Recent advances of persistent luminescence nanoparticles in bioapplications. Nanomicro Lett 12(1):70. https://doi.org/10.1007/s40820-020-0404-8
Maldiney T, Bessiere A, Seguin J et al (2014) The in vivo activation of persistent nanophosphors for optical imaging of vascularization, tumours and grafted cells. Nat Mater 13(4):418–426. https://doi.org/10.1038/nmat3908
Maldiney T, Lecointre A, Viana B et al (2011) Controlling electron trap depth to enhance optical properties of persistent luminescence nanoparticles for in vivo imaging. J Am Chem Soc 133(30):11810–11815. https://doi.org/10.1021/ja204504w
Maldiney T, Richard C, Seguin J et al (2011) Effect of core diameter, surface coating, and PEG chain length on the biodistribution of persistent luminescence nanoparticles in mice. ACS Nano 5(2):854–862. https://doi.org/10.1021/nn101937h
Bessiere A, Jacquart S, Priolkar K et al (2011) ZnGa2O4:Cr3+: a new red long-lasting phosphor with high brightness. Opt Express 19(11):101319–101317. https://doi.org/10.1364/OE.19.010131
Katayama Y, Kobayashi H, Tanabe S (2015) Deep-red persistent luminescence in Cr3+-doped LaAlO3 perovskite phosphor for in vivo imaging. Appl Phys Express 8(1). https://doi.org/10.7567/apex.8.012102
Maldiney T, Viana B, Bessière A et al (2013) In vivo imaging with persistent luminescence silicate-based nanoparticles. Opt Mater 35(10):1852–1858. https://doi.org/10.1016/j.optmat.2013.03.028
Maldiney T, Kaikkonen MU, Seguin J et al (2012) In vitro targeting of avidin-expressing glioma cells with biotinylated persistent luminescence nanoparticles. Bioconjug Chem 23(3):472–478. https://doi.org/10.1021/bc200510z
Rosticher C, Chanéac C, Viana B et al (eds) (2015) Red persistent luminescence and magnetic properties of nanomaterials for multimodal imaging. Proc.SPIE;
Sun M, Li Z-J, Liu C-L et al (2014) Persistent luminescent nanoparticles for super-long time in vivo and in situ imaging with repeatable excitation. J Lumin 145:838–842. https://doi.org/10.1016/j.jlumin.2013.08.070
Wang J, Li J, Yu J, Zhang H, Zhang B (2018) Large hollow cavity luminous nanoparticles with near-infrared persistent luminescence and tunable sizes for Tumor afterglow imaging and chemo-/photodynamic therapies. ACS Nano 12(5):4246–4258. https://doi.org/10.1021/acsnano.7b07606
Liu F, Yan W, Chuang YJ et al (2013) Photostimulated near-infrared persistent luminescence as a new optical read-out from Cr3+-doped LiGa5O8. Sci Rep 3:1554. https://doi.org/10.1038/srep01554
Kamimura S, Xu C-N, Yamada H, Terasaki N, Fujihala M (2014) Long-persistent luminescence in the near-infrared from Nd3+-doped Sr2SnO4 for in vivo optical imaging. Jpn J Appl Phys 53(9). https://doi.org/10.7567/jjap.53.092403
Liang L, Chen J, Shao K et al (2023) Controlling persistent luminescence in nanocrystalline phosphors. Nat Mater 22(3):289–304. https://doi.org/10.1038/s41563-022-01468-y
Hong G, Antaris AL, Dai H (2017) Near-infrared fluorophores for biomedical imaging. Nat Biomed Eng 1(1). https://doi.org/10.1038/s41551-016-0010
Soga K, Tokuzen K, Tsuji K et al (2010) NIR bioimaging: development of liposome-encapsulated, rare‐earth‐doped Y2O3 nanoparticles as fluorescent probes. Eur J Inorg Chem 2010(18):2673–2677. https://doi.org/10.1002/ejic.201000201
Zhang Y, Chen D, Wang W et al (2020) Long-lasting ultraviolet-A persistent luminescence and photostimulated persistent luminescence in Bi3+-doped LiScGeO4 phosphor. Inorg Chem Front 7(17):3063–3071. https://doi.org/10.1039/d0qi00578a
Zou Z, Tang X, Wu C et al (2018) How to tune trap properties of persistent phosphor: photostimulated persistent luminescence of NaLuGeO4:Bi3+,Cr3+ tailored by trap engineering. Mater Res Bull 97:251–259. https://doi.org/10.1016/j.materresbull.2017.09.011
Wang Z, Wang W, Zhou H et al (2016) Superlong and color-tunable red persistent luminescence and photostimulated luminescence properties of NaCa2GeO4F:Mn2+,Yb3+ phosphor. Inorg Chem 55(24):12822–12831. https://doi.org/10.1021/acs.inorgchem.6b02136
Shi J, Sun X, Zhu J, Li J, Zhang H (2016) One-step synthesis of amino-functionalized ultrasmall near infrared-emitting persistent luminescent nanoparticles for in vitro and in vivo bioimaging. Nanoscale 8(18):9798–9804. https://doi.org/10.1039/c6nr00590j
Lemański K, Babij M, Dereń PJ (2019) Upconversion emission of the GaN nanocrystals doped with rare earth ions. Solid State Sci 94:127–132. https://doi.org/10.1016/j.solidstatesciences.2019.06.005
Ding Z, He Y, Rao H et al (2022) Novel fluorescent probe based on rare-earth doped upconversion nanomaterials and its applications in early cancer detection. Nanomaterials (Basel) 12(11). https://doi.org/10.3390/nano12111787
Hong E, Liu L, Bai L et al (2019) Control synthesis, subtle surface modification of rare-earth-doped upconversion nanoparticles and their applications in cancer diagnosis and treatment. Mater Sci Eng C Mater Biol Appl 105:110097. https://doi.org/10.1016/j.msec.2019.110097
Zheng X, Kankala RK, Liu C-G et al (2021) Lanthanides-doped near-infrared active upconversion nanocrystals: Upconversion mechanisms and synthesis. Coordin Chem Rev 438. https://doi.org/10.1016/j.ccr.2021.213870
Wang Y, Zheng K, Song S et al (2018) Remote manipulation of upconversion luminescence. Chem Soc Rev 47(17):6473–6485. https://doi.org/10.1039/c8cs00124c
Liu F, Liang Y, Pan Z (2014) Detection of up-converted persistent luminescence in the near infrared emitted by the Zn3Ga2GeO8:Cr3+,Yb3+,Er3+ phosphor. Phys Rev Lett 113(17):177401. https://doi.org/10.1103/PhysRevLett.113.177401
Haase M, Schafer H (2011) Upconverting nanoparticles. Angew Chem Int Ed Engl 50(26):5808–5829. https://doi.org/10.1002/anie.201005159
Cheng Y, Sun K, Ge P (2018) Yb3+ and Er3+ co-doped ZnGa2O4:Cr3+ powder phosphors: combining green up-conversion emission and red persistent luminescence. Opt Mater 83:13–18. https://doi.org/10.1016/j.optmat.2018.05.048
Qin J, **ang J, Suo H et al (2019) NIR persistent luminescence phosphor Zn1.3Ga1.4Sn0.3O4:Yb3+,Er3+,Cr3+ with 980 nm laser excitation. J Mater Chem C 7(38):11903–11910. https://doi.org/10.1039/c9tc03882e
Ge P, Sun K, Li H et al (2020) Near-infrared up-converted persistent luminescence in Zn3Ga2SnO8:Cr3+,Yb3+,Er3+ nano phosphor for imaging. Optik 218. https://doi.org/10.1016/j.ijleo.2020.164944
Ge P, Sun K, Cheng Y (2019) Design and synthesis of up-converted persistent luminescence Zn3Ga2SnO8:Cr3+,Yb3+,Er3+ phosphor. Optik 188:200–204. https://doi.org/10.1016/j.ijleo.2019.05.011
Cheng Y, Sun K (2020) Up-conversion persistent luminescence of a 980 nm laser activated Zn3Ga2(GexSn1–x)O8:Yb,Er,Cr phosphors. J Fluoresc 30(5):1251–1259. https://doi.org/10.1007/s10895-020-02593-0
Yang J, Jiang R, Meng Y et al (2021) NIR-I/III afterglow induced by energy transfers between Er and Cr Codoped in ZGGO nanoparticles for potential bioimaging. J Am Ceram Soc 104(9):4637–4648. https://doi.org/10.1111/jace.17880
Xue Z, Li X, Li Y et al (2017) A 980 nm laser-activated upconverted persistent probe for NIR-to-NIR rechargeable in vivo bioimaging. Nanoscale 9(21):7276–7283. https://doi.org/10.1039/c6nr09716b
Li Z, Huang L, Zhang Y et al (2017) Near-infrared light activated persistent luminescence nanoparticles via upconversion. Nano Res 10(5):1840–1846. https://doi.org/10.1007/s12274-017-1548-9
Jia D (2006) Enhancement of long-persistence by Ce co-do** in CaS:Eu2+,Tm3+ red phosphor. J Electrochem Soc 153(11). https://doi.org/10.1149/1.2337087
Wu X, Zhang Y, Takle K et al (2016) Dye-sensitized core/active shell upconversion nanoparticles for optogenetics and bioimaging applications. ACS Nano 10(1):1060–1066. https://doi.org/10.1021/acsnano.5b06383
Hu L, Fan Y, Liu L et al (2017) Orthogonal multiplexed luminescence encoding with near-infrared rechargeable upconverting persistent luminescence composites. Adv Opt Mater 5(22). https://doi.org/10.1002/adom.201700680
Qiu X, Zhu X, Xu M et al (2017) Hybrid nanoclusters for near-infrared to near-infrared upconverted persistent luminescence bioimaging. ACS Appl Mater Interfaces 9(38):32583–32590. https://doi.org/10.1021/acsami.7b10618
Giordano L, Cai G, Seguin J et al (2023) Persistent luminescence induced by upconversion: an alternative approach for rechargeable bio-emitters. Adv Opt Mater 11(11). https://doi.org/10.1002/adom.202201468
Giordano L, Cai G, Seguin J et al (2023) Upconverted persistent luminescence in β-NaGd0.8Yb0.17Er0.03F4 and Zn1.33Ga1.335Sn0.33Cr0.005O4 associated nanoparticles. SPIE OPTO. SPIE. https://doi.org/10.1117/12.2651117
Chen X, Li Y, Huang K et al (2021) Trap energy upconversion-like near-infrared to near-infrared light rejuvenateable persistent luminescence. Adv Mater 33(15):e2008722. https://doi.org/10.1002/adma.202008722
** for upconversion-like trap energy transfer NIR persistent luminescence. Inorg Chem Front 10(7):2174–2188. https://doi.org/10.1039/d3qi00184a
Li T, Li Y, Yuan P, Ge D, Yang Y (2019) Efficient X-ray excited short-wavelength infrared phosphor. Opt Express 27(9):13240–13251. https://doi.org/10.1364/OE.27.013240
Westphal ER, Brown AD, Quintana EC et al (2021) Visible emission spectra of thermographic phosphors under x-ray excitation. Meas Sci Technol 32(9). https://doi.org/10.1088/1361-6501/abf222
Li S, Liu Y, Liu C et al (2017) Design, fabrication and characterization of nanocaged 12CaO·7Al2O3:Tb3+ photostimulable phosphor for high-quality X-ray imaging. Mater Des 134:1–9. https://doi.org/10.1016/j.matdes.2017.08.027
Waetzig GR, Horrocks GA, Jude JW et al (2018) Ligand-mediated control of dopant oxidation state and X-ray excited optical luminescence in Eu-doped LaOCl. Inorg Chem 57(10):5842–5849. https://doi.org/10.1021/acs.inorgchem.8b00234
Kuang Y, Pratx G, Sun C, Carpenter C, **ng L (2011) TU-A-301-08: X-ray stimulated fluorescence for breast imaging. Med Phys 38(6Part28):3746. https://doi.org/10.1118/1.3613098
Naczynski DJ, Sun C, Turkcan S et al (2015) X-ray-induced shortwave infrared biomedical imaging using rare-earth nanoprobes. Nano Lett 15(1):96–102. https://doi.org/10.1021/nl504123r
**ong P, Peng M (2019) (INVITED) Recent advances in ultraviolet persistent phosphors. Optical Materials: X 2:100022. https://doi.org/10.1016/j.omx.2019.100022
Richard C, Viana B (2022) Persistent x-ray-activated phosphors: mechanisms and applications. Light Sci Appl 11(1):123. https://doi.org/10.1038/s41377-022-00808-6
Ma L, Zou X, Bui B et al (2014) X-ray excited ZnS:Cu,Co afterglow nanoparticles for photodynamic activation. Appl Phys Lett 105(1). https://doi.org/10.1063/1.4890105
Xue Z, Li X, Li Y et al (2017) X-ray-activated near-infrared persistent luminescent probe for deep-tissue and renewable in vivo bioimaging. ACS Appl Mater Interfaces 9(27):22132–22142. https://doi.org/10.1021/acsami.7b03802
Rosticher C, Viana B, Laurent G, Le Griel P, Chanéac C (2015) Insight into CaMgSi2O6:Eu2+,Mn2+,Dy3+ nanoprobes: influence of chemical composition and crystallinity on persistent red luminescence. Eur J Inorg Chem 2015(22):3681–3687. https://doi.org/10.1002/ejic.201500257
Kong J, Zheng W, Liu Y et al (2015) Persistent luminescence from Eu3+ in SnO2 nanoparticles. Nanoscale 7(25):11048–11054. https://doi.org/10.1039/c5nr01961c
Zhuang Y, Ueda J, Tanabe S (2013) Tunable trap depth in Zn(Ga1 – xAlx)2O4:Cr,Bi red persistent phosphors: considerations of high-temperature persistent luminescence and photostimulated persistent luminescence. J Mater Chem C 1(47). https://doi.org/10.1039/c3tc31462f
Lin XH, Song L, Chen S et al (2017) Kiwifruit-like persistent luminescent nanoparticles with high-performance and in situ activable near-infrared persistent luminescence for long-term in vivo bioimaging. ACS Appl Mater Interfaces 9(47):41181–41187. https://doi.org/10.1021/acsami.7b13920
Chen H, Sun X, Wang GD et al (2017) LiGa5O8:Cr-based theranostic nanoparticles for imaging-guided X-ray induced photodynamic therapy of deep-seated tumors. Mater Horiz 4(6):1092–1101. https://doi.org/10.1039/C7MH00442G
Yakunin S, Sytnyk M, Kriegner D et al (2015) Detection of X-ray photons by solution-processed organic-inorganic perovskites. Nat Photonics 9(7):444–449. https://doi.org/10.1038/nphoton.2015.82
Liu B-M, Zou R, Lou S-Q et al (2021) Low-dose x-ray-stimulated LaGaO3:Sb,Cr near-infrared persistent luminescence nanoparticles for deep-tissue and renewable in vivo bioimaging. Chem Eng J 404. https://doi.org/10.1016/j.cej.2020.127133
Zheng H, Liu L, Li Y et al (2023) X-ray excited Mn2+-doped persistent luminescence materials with biological window emission for in vivo bioimaging. J Rare Earth. https://doi.org/10.1016/j.jre.2023.01.004
Peng M, Yin X, Tanner PA, Brik MG, Li P (2015) Site occupancy preference, enhancement mechanism, and thermal resistance of Mn4+ red luminescence in Sr4Al14O25: Mn4+ for warm WLEDs. Chem Mater 27(8):2938–2945. https://doi.org/10.1021/acs.chemmater.5b00226
Zhou Z, Zhou N, **a M, Yokoyama M, Hintzen HT (2016) Research progress and application prospects of transition metal Mn4+-activated luminescent materials. J Mater Chem C 4(39):9143–9161. https://doi.org/10.1039/c6tc02496c
Du J, Poelman D (2019) Near-infrared persistent luminescence in Mn4+ doped perovskite type solid solutions. Ceram Int 45(7):8345–8353. https://doi.org/10.1016/j.ceramint.2019.01.142
Ding S, Guo H, Feng P, Ye Q, Wang Y (2020) A new near-infrared long persistent luminescence material with its outstanding persistent luminescence performance and promising multifunctional application prospects. Adv Opt Mater 8(18). https://doi.org/10.1002/adom.202000097
Du J, Li K, Van Deun R, Poelman D, Lin H (2021) Near-infrared persistent luminescence and trap reshuffling in Mn4+ doped alkali‐earth metal tungstates. Adv Opt Mater 10(2). https://doi.org/10.1002/adom.202101714
Wei Y, Gong C, Zhao M et al (2022) Recent progress in synthesis of lanthanide-based persistent luminescence nanoparticles. J Rare Earth 40(9):1333–1342. https://doi.org/10.1016/j.jre.2022.05.016
Song L, Lin XH, Song XR et al (2017) Repeatable deep-tissue activation of persistent luminescent nanoparticles by soft X-ray for high sensitivity long-term in vivo bioimaging. Nanoscale 9(8):2718–2722. https://doi.org/10.1039/c6nr09553d
Li Y, Gecevicius M, Qiu J (2016) Long persistent phosphors–from fundamentals to applications. Chem Soc Rev 45(8):2090–2136. https://doi.org/10.1039/c5cs00582e
Hu Y, Li X, Wang X et al (2020) Greatly enhanced persistent luminescence of YPO4:Sm3+ phosphors via Tb3+ incorporation for in vivo imaging. Opt Express 28(2):2649–2660. https://doi.org/10.1364/OE.384678
Ou X, Qin X, Huang B et al (2021) High-resolution X-ray luminescence extension imaging. Nature 590(7846):410–415. https://doi.org/10.1038/s41586-021-03251-6
Jiang S, Lin J, Huang P (2022) Nanomaterials for NIR-II Photoacoustic Imaging. Adv Healthc Mater e2202208. https://doi.org/10.1002/adhm.202202208
Pei P, Chen Y, Sun C et al (2021) X-ray-activated persistent luminescence nanomaterials for NIR-II imaging. Nat Nanotechnol 16(9):1011–1018. https://doi.org/10.1038/s41565-021-00922-3
Liang YJ, Liu F, Chen YF et al (2016) New function of the Yb3+ ion as an efficient emitter of persistent luminescence in the short-wave infrared. Light Sci Appl 5(7):e16124. https://doi.org/10.1038/lsa.2016.124
Ding D, Li S, Xu H et al (2021) X-ray-activated simultaneous near-infrared and short-wave infrared persistent luminescence imaging for long-term tracking of drug delivery. ACS Appl Mater Interfaces 13(14):16166–16172. https://doi.org/10.1021/acsami.1c02372
Zheng S, Shi J, Fu X et al (2020) X-ray recharged long afterglow luminescent nanoparticles MgGeO3:Mn2+,Yb3+,Li+ in the first and second biological windows for long-term bioimaging. Nanoscale 12(26):14037–14046. https://doi.org/10.1039/c9nr10622g
Sengar P, Juarez P, Verdugo-Meza A et al (2018) Development of a functionalized UV-emitting nanocomposite for the treatment of cancer using indirect photodynamic therapy. J Nanobiotechnol 16(1):19. https://doi.org/10.1186/s12951-018-0344-3
Cai H, Song Z, Liu Q (2021) Infrared-photostimulable and long-persistent ultraviolet-emitting phosphor LiLuGeO4:Bi3+,Yb3+ for biophotonic applications. Mater Chem Front 5(3):1468–1476. https://doi.org/10.1039/d0qm00932f
Liu L, Yu K, Ming L et al (2022) A novel Gd-based phosphor NaGdGeO4:Bi3+,Li+ with super-long ultraviolet-A persistent luminescence. J Rare Earth 40(9):1424–1431. https://doi.org/10.1016/j.jre.2021.04.017
Yin X, Zhong H, Liu L et al (2023) X-ray-activated Bi3+/Pr3+ co-doped LiYGeO4 phosphor with UV and NIR dual-emissive persistent luminescence. J Rare Earth. https://doi.org/10.1016/j.jre.2023.03.008
Yu N, Li Y, Li Z, Han G (2018) The bottom-up synthesis and applications of persistent luminescence nanoparticles. Sci China Chem 61(7):757–758. https://doi.org/10.1007/s11426-018-9263-9
Zhou Q, Xu M, Feng W, Li F (2021) Quantum Yield measurements of Photochemical reaction-based afterglow luminescence materials. J Phys Chem Lett 12(39):9455–9462. https://doi.org/10.1021/acs.jpclett.1c02715
Poon W, Zhang YN, Ouyang B et al (2019) Elimination pathways of nanoparticles. ACS Nano 13(5):5785–5798. https://doi.org/10.1021/acsnano.9b01383
Lecuyer T, Durand MA, Volatron J et al (2020) Degradation of ZnGa2O4:Cr3+ luminescent nanoparticles in lysosomal-like medium. Nanoscale 12(3):1967–1974. https://doi.org/10.1039/c9nr06867h
Teston E, Richard S, Maldiney T et al (2015) Non-aqueous sol-gel synthesis of ultra small persistent luminescence nanoparticles for near-infrared in vivo imaging. Chemistry 21(20):7350–7354. https://doi.org/10.1002/chem.201406599
Lecuyer T, Seguin J, Balfourier A et al (2022) Fate and biological impact of persistent luminescence nanoparticles after injection in mice: a one-year follow-up. Nanoscale 14(42):15760–15771. https://doi.org/10.1039/d2nr03546d
Ramirez-Garcia G, Gutierrez-Granados S, Gallegos-Corona MA et al (2017) Long-term toxicological effects of persistent luminescence nanoparticles after intravenous injection in mice. Int J Pharm 532(2):686–695. https://doi.org/10.1016/j.ijpharm.2017.07.015
Jiang Y, Li Y, Richard C, Scherman D, Liu Y (2019) Hemocompatibility investigation and improvement of near-infrared persistent luminescent nanoparticle ZnGa2O4:Cr3+ by surface PEGylation. J Mater Chem B 7(24):3796–3803. https://doi.org/10.1039/c9tb00378a
Funding
This work was supported in part by the China Postdoctoral ScienceFoundation (No.2022M711438), Natural Science Foundation of Shan-dong Province (ZR2020ME045 and ZR2020MEO46), “New Universities20” Foundation of **an (Grant No.2021GXRCO99, T202204), Science andTechnology Program of University of **an (No.XKY2016).
Author information
Authors and Affiliations
Contributions
YQ.L. wrote the main manuscript text and prepared Figs. 1, 2, 3, 4, 5 and 6 with Table 1, and 2. JK.L. proposed the general structure of the article and provided writing guidance. JQ.XH. reviewed the manuscript. ZM.L. supervised the entire process.
Corresponding authors
Ethics declarations
Ethical Approval
Not available.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Li, J., **ahou, J. et al. Recent Advances in NIR or X-ray Excited Persistent Luminescent Materials for Deep Bioimaging. J Fluoresc (2023). https://doi.org/10.1007/s10895-023-03513-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-023-03513-8