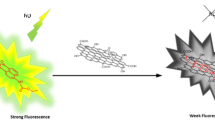
Abstract
A naphthalene diimide dye with two side amine arm was prepared. Uv–Vis and fluorescence spectroscopic techniques are used for their photophysical and solvatochromic characteristics in different solvents. The Lippert-Mataga plot for naphthalene diimide demonstrated a negative linear dependence by increasing polarity. Results showed naphthalene diimide is more polar in the ground than in the excited state. A quenching study was conducted for interacting the naphthalene diimide as a fluorophore and graphene oxide as a quencher. Fluorescence quenching-based platforms in nanoscale have been used in sensing systems. Raman, FTIR, Uv–Vis and fluorescence spectroscopic techniques were used to study the quenching mechanism. The results indicated that graphene plays an effective quencher against the naphthalene diimide, with a quenching efficiency 91%. The Stern–Volmer analysis results show a mix of static and dynamic quenching mechanisms. The binding constant of the quencher-fluorophore and the number of binding sites have been reported. Thermodynamic parameters of their interaction were evaluated. The negative values of the Gibbs free energy confirm that the complexation process is spontaneous. Meanwhile, the positive entropy value confirms that the favorable pathway process.












Similar content being viewed by others
Availability of Data and Material/Data Availability
Not applicable.
References
Lee XJ, Hiew BYZ, Lai KC, Lee LY, Gan S, Thangalazhy-Gopakumar S, Rigby S (2019) Review on graphene and its derivatives: Synthesis methods and potential industrial implementation. J Taiwan Inst Chem Eng 98:163–180. https://doi.org/10.1016/j.jtice.2018.10.028
Zhu Y, Murali S, Cai W, Li X, Suk JW, Potts JR, Ruoff RS (2010) Graphene and graphene oxide: synthesis, properties, and applications. Adv Materials 22:3906–3924. https://doi.org/10.1002/adma.201001068
Geim AK, Novoselov KS et al (2007) The rise of graphene. Nat Mater 6:183–191. https://doi.org/10.1038/nmat1849
Shahdeo D, Roberts A, Abbineni N, Gandhi S (2020) Graphene based sensors. Compr Anal Chem 91:175–199. https://doi.org/10.1016/bs.coac.2020.08.007
Lu CH, Yang H (2009) A graphene platform for sensing biomolecules. Angew Chem 48:4785–4797. https://doi.org/10.1002/anie.200901479
Narvaez E, Merkoci A et al (2012) Graphene oxide as an optical biosensing platform. Adv Funct Mater 24:3298–3308. https://doi.org/10.1002/adma.201200373
Loh KP, Bao Q, Eda G, Chhowalla M (2010) Graphene oxide as a chemically tunable platform for optical applications. Nat Chem 2:1015–10244797. https://doi.org/10.1038/nchem.907
Zhau Y, Gang X et al (2016) Review on the graphene based optical fiber chemical and biological sensor. Sens Actuators B Chem 231:324–340. https://doi.org/10.1016/j.snb.2016.03.026
Gao X-G, Cheng L-X, Jiang W-S, Li X-K, **ng F (2021) Graphene and its derivatives-based optical sensors. Front Chem 9:615164. https://doi.org/10.3389/fchem.2021.615164
Ifrah Z, Shah Rukh A, Muhammad Nauman S, Maryam S, Rahat U (2022) Fluorescence quenching of graphene quantum dots by chloride ions: A potential optical biosensor for cystic fibrosis. Front Mater 9:857432. https://doi.org/10.3389/fmats.2022.857432
Chen H, Gao Q, Li J, Jm L (2016) Graphene materials-based chemiluminescence for sensing. J Photochem Photobiol, C 27:54–71. https://doi.org/10.1016/j.jphotochemrev.2016.04.0
Anas NAA, Fen YW, Omar NAS, Daniyal WMEMM, Ramdzan NSM, Saleviter S (2019) Development of graphene quantum dots-based optical sensor for toxic metal ion detection. Sensors 19:3850. https://doi.org/10.3390/s19183850
Kasry A, Ardakani AA, Tulevski GS, Menges B, Copel M, Vyklicky L (2012) Highly efficient fluorescence quenching with graphene. J Phys Chem C 116:2858–2862. https://doi.org/10.1021/jp207972f
**ao X, Zhang Y, Zhou L, Li B, Gu L (2022) Photoluminescence and fluorescence quenching of graphene oxide: A review. Nanomaterials 12:2444. https://doi.org/10.3390/nano12142444
Liu J, **a S, Liu D, Hou J, Suo H, Cheng F (2020) Multifunctional naphthalene diimide-based coordination polymers: Photochromism and solventchromism. Dyes Pigm 177:108269. https://doi.org/10.1016/j.dyepig.2020.108269
Bhosale SV, Al-Kobaisi M, Jadhav RW, Morajkar PP, Jones LA, George S (2021) Naphthalene diimides: perspectives and promise. Chem Soc Rev 50:9845–9998. https://doi.org/10.1039/D0CS00239A
Lasitha P (2020) Radical anion formation exhibiting “turn-on” fluorescence sensing of hydrazine using a naphthalene diimide (NDI) derivative with a donor-acceptor-donor (DAD) molecular structure. Photochem Photobiol Sci 19:1603–1612. https://doi.org/10.1039/D0PP00232A
Ling QH, Zhu JL, Qin Y, Xu L (2020) Naphthalene diimide-and perylene diimide-based supramolecular cages. Naphthalene diimide-and perylene diimide-based supramolecular cages. Mater Chem Front 4:3176–3189. https://doi.org/10.1039/D0QM00540A
Gharagozlou M, Rouhani S (2022) A New reusable mercury-sensitive turn-on nano-chemosensor based on functionalized CoFe2O4@ SiO2 magnetic nanocomposite. Prog Color Colorants Coat 15:75–85. https://doi.org/10.30509/PCCC.2021.166735.1091
Seraj S, Rouhani S (2017) A fluorescence quenching study of naphthalimide dye by graphene mechanism and thermodynamic properties. J Fluoresc 27:1877–1883. https://doi.org/10.1007/s10895-017-2126-y
Seraj S, Rouhani S, Faridbod F (2019) Naphthalimide-based optical turn-on sensor for monosaccharide recognition using boronic acid receptor. RSC Adv 9:17933–17940. https://doi.org/10.1039/C9RA01757G
Seraj S, Rouhani S (2021) Synthesis and fluorescence quenching mechanism of novel naphthalimide derivative by nanographene oxide. Chem Phys Lett 780:138895. https://doi.org/10.1016/j.cplett.2021.138895
Joseph RL (2006) Principles of fluorescence spectroscopy, 3rd edn. Springer New York, NY. https://doi.org/10.1007/978-0-387-46312-4
Lippert E (1957) Spektroskopische Bestimmung desDipolmomentes aromatischer Verbidungen im ersten angeregten Singulettzustand. Z Elektrochem Ber Bunsenges Phys Chem 61:962–975. https://doi.org/10.1002/bbpc.19570610819
Mataga N, Kaifu Y, Koizumi M (1956) Solvent effects upon fluorescence spectra and the dipolmoments of excited molecules. Bull Chem Soc Jpn 29:465–470. https://doi.org/10.1246/bcsj.29.465
Manivannan C, Renganathan R (2011) A study on the fluorescence quenching of 9-Aminoacridine by certain antioxidants. J Lumin 131:2365–2371. https://doi.org/10.1016/j.jlumin.2011.05.050
Cui F, Zhang Q, Yao X, Luo H, Yang Y, Qin L, Qu G, Lu Y (2008) The investigation of the interaction between 5-Iodouracil and human serum albumin by spectroscopic and modeling methods and determination of protein by synchronous fluorescence technique. Pestic Biochem Physiol 90(2):126–134. https://doi.org/10.1016/j.pestbp.2007.11.002
Laws WR, Contino PB (1992) Fluorescence quenching studies: Analysis of nonlinear Stern-Volmer data. Methods Enzymol 210:448–463. https://doi.org/10.1016/0076-6879(92)10023-7
Swathi RS, Sebastian KL (2008) Resonance energy transfer from a dye molecule to graphene. J Chem Phys 129:054703. https://doi.org/10.1063/1.2956498
Swathi RS, Sebastian KL (2009) Long range resonance energy transfer from a dye molecule to graphene has (distance)− 4 dependence. J Chem Phys 130:086101. https://doi.org/10.1063/1.3077292
Paredes JI, Villar-Rodil S, Martínez-Alonso A, Tascon JM (2008) Graphene oxide dispersions in organic solvents. Langmuir 24:10560–10564. https://doi.org/10.1021/la801744a
Lakowicz JR (2002) Topics in fluorescence spectroscopy, vol 7. Plenum Press, New York. https://doi.org/10.1007/b112909
Airinei A, Tigoianu RI, Rusu E, Dorohoi DO (2011) Fluorescence quenching of anthracene by nitroaromatic compounds. Dig J Nanomater Biostructures 6(3):1265–1272
Galindo F, Isabel Burguete M, Gavara R, Luis SV (2006) Fluorescence quenching in organogel as a reaction medium. J Photochem Photobiol A Chem 178(1):57–61. https://doi.org/10.1016/j.jphotochem.2005.06.021
Acknowledgements
We want to thank Institute for Color Science and Technology, Tehran-Iran, for the facilities and materials to complete this project. Also, thank the Center of Excellence of "The Institute for Color Science and Technology" for their spiritual support.
Author information
Authors and Affiliations
Contributions
MM performed the experimental data acquisition and interpretation of the results. SR and PZ designed and wrote the entire manuscript. All authors participated in discussing the results and approved the final draft of the manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mahdiani, M., Rouhani, S. & Zahedi, P. Synthesis, Solvatochromism and Fluorescence Quenching Studies of Naphthalene Diimide Dye by Nano graphene oxide. J Fluoresc 33, 2003–2014 (2023). https://doi.org/10.1007/s10895-023-03197-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-023-03197-0