Abstract
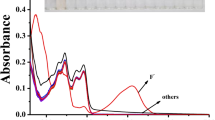
Upon the Schiff base condensation reaction of imidazo[1,2-a]pyridine-2-carbohydrazide and 2,5-dihydroxybenzaldehyde, a bimodal colorimetric and fluorescent chemosensor 1o for assaying fluoride (F−) in DMSO was synthesized. The characterization of 1o structure was obtained by 1H NMR, 13C NMR and MS.The structure of 1o was characterized by 1H NMR, 13C NMR and MS. Under the presence of various anions, 1o could be applied for naked-eye and fluorescent detection of F− (naked eye: colorless to yellow; fluorescence: dark to green) and displayed promising performance, such as high selectivity and sensitivity, as well as a low detection limit. Upon calculation, the detection limit of chemosensor 1o for F− was 193.5 nM, which is well below the allowed maximum value of F− (1.5 mg/L) by WHO. As the intermolecular proton transfer mechanism induced "turn-on" fluorescent signal and naked-eye color change of F− to 1o through deprotonation effect, which was confirmed by Job's plot curve, mass spectrometry and 1H NMR titration. Alternatively, the chemosensor 1o can be effectively manufactured into test strips to detect fluoride in solid state, which is user-friendly with no additional equipment required.
Graphical Abstract









Similar content being viewed by others
Availability of Data and Material
All data generated or analysed during this study are included in this published article (and its supplementary information files).
Code Availability
Not applicable.
References
Gale PA, Caltagirone C (2018) Fluorescent and colorimetric sensors for anionic species. Coord Chem Rev 354:2–27
Qu Y, Hua J, Tian H (2010) Colorimetric and ratiometric red fluorescent chemosensor for fluoride ion based on diketopyrrolopyrrole. Org Lett 12:3320–3323
Tang L, Zhou P, Zhang Q, Huang Z, Zhao J, Cai M (2013) A simple quinoline derivatized thiosemicarbazone as a colorimetic and fluorescent sensor for relay recognition of Cu2+ and sulfide in aqueous solution. Inorg Chem Commun 36:100–104
Mohammadi A, Jabbari J (2016) Simple naked-eye colorimetric chemosensors based on Schiff-base for selective sensing of cyanide and fluoride ions. Can J Chem 94:631–636
Mohammadi A, Kianfar M (2018) A simple colorimetric chemosensor with highly performance for detection of cyanide and copper ions and its practical application in real samples. J Photochem Photobiol A 367:22–31
Dong M, Peng Y, Dong YM, Tang N, Wang YW (2012) A selective, colorimetric, and fluorescent chemodosimeter for relay recognition of fluoride and cyanide anions based on 1, 1′-binaphthyl scaffold. Org Lett 14:130–133
**ao N, **e L, Zhi X, Fang C (2018) A naphthol-based highly selective fluorescence turn-on and reversible sensor for Al(III) ion. Inorg Chem Commun 89:13–17
Lin ZH, Ou SJ, Duan CY, Zhang BG, Bai ZP (2006) Naked-eye detection of fluoride ion in water: a remarkably selective easy-to-prepare test paper. Chem Commun 6:624–626
Kim TH, Swager TM (2003) A fluorescent self-amplifying wavelength-responsive sensory polymer for fluoride ions. Angew Chem Int Ed 42:4803–4806
Zhang JF, Zhou Y, Yoon J, Kim J (2011) Recent progress in fluorescent and colorimetric chemosensors for detection of precious metal ions (silver, gold and platinum ions). Chem Soc Rev 40:3416–3429
Cao XW, Lin WY, Yu QX, Wang JJ (2011) Ratiometric sensing of fluoride anions based on a BODIPY-coumarin platform. Org Lett 13:6089–6101
Yu MM, Xu J, Peng C, Li ZX, Liu CX, Wei LH (2016) A novel colorimetric and fluorescent probe for detecting fluoride anions: from water and toothpaste samples. Tetrahedron 72:273–278
Ozsvath DL (2008) Fluoride and environmental health:a review. Rev Environ Sci Biotechnol 8:59–79
Peng BX, Wu DS, Lai JH, **ao HY, Li P (2012) Simultaneous determination of halogens (F, Cl, Br, and I) in coal using pyrohydrolysis combined with ion chromatograph. Fuel 94:629–631
Konieczka P, Zygmunt B, Namiesnik J (2000) Comparison of fluoride ion-selective electrode based potentiometric methods of fluoride determination in human urine. Bull Environ Contam Toxicol 64:794–803
Liu BW, Huang ZC, Liu JW (2016) Boosting the oxidase mimicking activity of nanoceria by fluoride cap**:rivaling protein enzymes and ultrasensitive F- detection. Nanoscale 8:13562–13567
Moldoveanu SC, Kiser M (2007) Gas chromatography/mass spectrometry versus liquid chromatography/fluorescence detection in the analysis of phenols in mainstream cigarette smoke. J Chromatogr A 1141:90–97
Lee JJ, Park GJ, Kim YS, Lee SY, Lee HJ, Noh I, Kim C (2015) A water-soluble carboxylic-functionalized chemosensor for detecting Al3+ in aqueous media and living cells: Experimental and theoretical studies. Biosens Bioelectron 69:226–229
Montoya LA, Pluth MD (2012) Selective turn-on fluorescent probes for imaging hydrogen sulfide in living cells. Chem Commun 48:4767–4769
Park GJ, Jo HY, Ryu KY, Kim C (2014) A new coumarin-based chromogenic chemosensor for the detection of dual analytes Al3+ and F-. RSC Adv 4:63882–63890
Sen B, Mukherjee M, Banerjee S, Pal S, Chattopadhyay P (2015) A rhodamine-based ‘turn-on’ Al3+ ion-selective reporter and the resultant complex as a secondary sensor for F- ion are applicable to living cell staining. Dalton Trans 44:8708–8717
Datta BK, Thiyagarajan D, Ramesh A, Das G (2015) A sole multi-analyte receptor responds with three distinct fluorescence signals:traffic signal like sensing of Al3+, Zn2+ and F-. Dalton Trans 44:13093–13099
Ghosh K, Panja S, Sarkar T (2015) Rhodamine-linked pyridyl thiourea as a receptor for selective recognition of F-, Al3+ and Ag+ under different conditions. Supramol Chem 27:490–500
Kim YS, Lee JJ, Choi YW, You GR, Nguyen LT, Noh I, Kim C (2016) Simultaneous bioimaging recognition of cation Al3+ and anion F- by a fluorogenic method. Dye Pigment 129:43–53
Liu HY, Zhang BB, Tan CY, Liu F, Jk C, Tan Y, Jiang YY (2016) Simultaneous bioimaging recognition of Al3+ and Cu2+ in living-cell, and further detection of F- and S2- by a simple fluorogenic benzimidazole-based chemosensor. Talanta 161:309–319
Kim KB, Kim H, Song EJ, Kim S, Noh I, Kim C (2013) A cap-type Schiff base acting as a fluorescence sensor for zinc(ii) and a colorimetric sensor for iron(ii), copper(ii), and zinc(ii) in aqueous media. Dalton Trans 42:16569–16577
Lee SA, You GR, Choi YW, Jo HY, Kim AR, Noh I, Kim SJ, Kim Y, Kim C (2014) A new multifunctional Schiff base as a fluorescence sensor for Al3+ and a colorimetric sensor for CN− in aqueous media: an application to bioimaging. Dalton Trans 43:6650–6659
Jana S, Dalapati S, Alam MdA, Guchhait N (2012) Spectroscopic, colorimetric and theoretical investigation of salicylidene hydrazine based reduced Schiff base and its application towards biologically important anions. Spectrochim Acta A 92:131–136
Prabhu S, Saravanamoorthy S, Ashok M, Velmathi S (2012) Colorimetric and fluorescent sensing of multi metal ions and anions by salicyladimine based receptors. J Lumin 132:979–986
Zhang P, Shi BB, Wei TB, Zhang YM, Lin Q, Yao H, You YM (2013) A naphtholic Schiff base for highly selective sensing of cyanide via different channels in aqueous solution. Dyes Pigm 99:857–862
Bose P, Ghosh P (2010) Visible and near infrared sensing of fluoride by indole conjugated urea/thiourea ligands. Chem Commun 46:2962–2964
Saravanakumar D, Devaraj S, Lyyampillai S, Mohandoss K, Kandaswamy M (2008) Schiff base’s phenol-hydrazone derivatives as colorimetric chemosensors for fluoride ions. Tetrahedron Lett 49:127–132
Das AK, Goswami S (2017) 2-Hydroxy-1-napthaldehyde: A versatile building block for the development of sensors in supramolecular chemistry and molecular recognition. Sens Actuators B 245:1062–1125
Li Z, Wang SJ, **ao LW, Li XL, Shao XN, **g XM, Peng XX, Ren LL (2018) An efficient colorimetric probe for fluoride ion based on schiff base. Inorg Chim Acta 476:7–11
Li XM, Zhang Y, Chen AQ, Zhang BH, Zhang B, Song J (2017) A ferrocene-based organogel with multi-stimuli properties and applications in naked-eye recognition of F- and Al3+. RSC Adv 7:37105–37111
Alreja P, Kaur N (2019) “Test kit” of chromogenic and ratiometric 1,10-phenanthroline based chemosensor for the recognition of F- and CN- ions. Inorg Chem Commun 110:107600
Mondal A, Banerjee P (2021) Chromofluorogenic sensory probe for ppb level recognition of hazardous F-: Proposition towards Hg2+ mediated logic gate simulator. J Fluorine Chem 246:109783
Jeong HY, Lee SY, Kim C (2017) Furan and Julolidine-based “turn-on” fluorescence chemosensor for detection of f- in a near-perfect aqueous solution. J Fluoresc 27:1457–1466
Li LH, Zhang M, Ding L, Ren GD, Hou XY, Liu W, Wang HJ, Wang B, Yan LL, Diao HP (2021) Ultrafast fluorescent probe with near-infrared analytical wavelength for fluoride ion detection in real samples. Spectrochim Acta Part A Mol Biomol Spectrosc 252:119518
Bhaskar R, Mageswari N, Sankar D, Vinoth KG (2022) Fluorescence chemosensor for fluoride ion using quinoline-derived probe: Molecular logic gate application. Mater Lett 327:133040
Acknowledgements
The authors are grateful for financial support from the National Natural Science Foundation of China (41867053), the project of the Science Funds of Jiangxi Education Office (GJJ190597).
Funding
This work was supported by the National Natural Science Foundation of China (41867052) and the project of the Science Funds of Jiangxi Education Office (GJJ190597).
Author information
Authors and Affiliations
Contributions
All authors contributed to the design and synthesis of this study. All authors performed the experimental material preparation, data testing and analysis of the results. The first draft of the manuscript was written by **n Mei and all authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
All authors given consent for participation.
Consent for Publication
All authors agreed to publish.
Conflicts of Interest
No conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mei, X., Li, H. & Pu, S. A Handy Chemical Sensor Based on Benzaldehyde and Imidazo[1,2-a]pyridine Mixture for Naked-eye Colorimetric and Fluorescent Detection of F−. J Fluoresc 33, 2381–2390 (2023). https://doi.org/10.1007/s10895-023-03195-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-023-03195-2