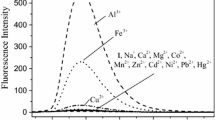
Abstract
A new and innovative fluorescent structure was constructed on Cyclotriveratrylene affiliated to Dansyl chloride (DNSC) and was used to detect Cr (III) and Fe (III) among the various cations by using spectrofluorimetric method. The characterization of the new compound was carried out using the 1H-NMR, 13C-NMR, and ESI–MS techniques. The interaction and role of DNSC-CTV with cations was reviewed. A change in the spectra of absorption directed to the conclusion that there is substantial interaction of Cr (III) and Fe (III) with DNSC-CTV. Furthermore the interaction of the ligand DNSC-CTV with the metal ions Chromium (III) and Iron (III) showed quenching in the emission spectra. Quantum yield of the complexes were calculated and the stern volmer analysis was done to deduce the quenching mechanism of fluorescence to being either static or dynamic. The molecule DNSC-CTV was further studied with the help of computational methods such as molecular docking to study the binding interactions and properties of the molecule.












Similar content being viewed by others
Code Availability
Hex 8.0 http://hex.loria.fr
Avogadro https://avogadro.cc/
References
Timothy E (2017) Glass, 8.01 - Introduction,Editor(s): Jerry L. Atwood,Comprehensive Supramolecular Chemistry II. Elsevier
**e XS (1996) Acc Chem Res 29:598
Goodwin PM, Ambrose WP, Keller RA (1996) Acc Chem Res 29:607
Orrit M, Bernard J (1990) Phys Rev Lett 65:2716
Mets U, Rigler RJ (1994) Fluoresc 4:259
Moerner WE, Basche TA (1996) Chem., Int. Ed. Engl.1993,32, 457. Moerner, W. E.Acc. Chem Res 29 563
Yeung ES (1994) Acc Chem Res 27:209
Aragoni MC et al (2007) "New fluorescent chemosensors for heavy metal ions based on functionalized pendant arm derivatives of 7-anthracenylmethyl-1, 4, 10-trioxa-7, 13-diazacyclopentadecane."Inorg Chem 46 19 (2007): 8088–8097
Misra A, Shahid M, Srivastava P et al (2011) J Incl Phenom Macrocycl Chem 69:119. https://doi.org/10.1007/s10847-010-9821-5
Chen QY, Chuan-Feng C (2005) "A new Hg2+-selective fluorescent sensor based on a dansyl amide-armed calix [4]-aza-crown."Tetrahedron Lett 46 1: 165–168
Talanova GG et al (1999) "A calixarene-based fluorogenic reagent for selective mercury (II) recognition."Analyt Chemistry 71 15: 3106–3109
Talanova GG et al (2005) "Novel fluorogenic calix [4] arene-bis (crown-6-ether) for selective recognition of thallium (I)."Chem Commun 45: 5673–5675
Schönefeld K, Ludwig R, Feller KH (2006) "Fluorescence studies of host–guest interaction of a dansyl amide labelled Calix [6] arene." J Fluores 16 3 449–454
Bhatt KD et al (2012) "Calix receptor edifice; scrupulous turn off fluorescent sensor for Fe (III), Co (II) and Cu (II)." J Fluores 22.6: 1493–1500
Formic M et al (2012) "New fluorescent chemosensors for metal ions in solution." Coor Chem Rev 256 1–2: 170–192
Hardie MJ (2010) "Recent advances in the chemistry of cyclotriveratrylene."Chem Soc Rev 39.2: 516–527
Fix OK, Kowdley KV (2008) Hereditaryhemochromatosis. MinervaMed 99:605-617
Office of Dietary Supplements (2016) US National Institutes of Health. Retrieved 26 June 2016
Vincent JB (2015) Is the Pharmacological Mode of Action of Chromium(III) as a Second Messenger? Biol Trace Elem Res 166(1):7–12
Chakrabarti A et al (2005) "Convenient synthesis of selectively substituted tribenzo [a, d, g] cyclononatrienes." Tetrahedron 61 52: 12323–12329
Talanova GG, Talanov VS (2010) "Dansyl-containing fluorogenic calixarenes as optical chemosensors of hazardous metal ions: a mini-review."Supramole Chem 22 11–12: 838–852
Patra S, Parimal P (2009) "Synthesis, characterization, electrochemistry and ion-binding studies of ruthenium (II) bipyridine receptor molecules containing calix [4] arene-azacrown as ionophore." Dalton Transact 40 : 8683–8695
Boricha VP et al (2009) "Synthesis, Characterisation, Electrochemistry and Ion‐Binding Studies of Ruthenium (II) and Rhenium (I) Bipyridine/Crown Ether Receptor Molecules." Euro J Inorg Chem 9: 1256–1267
Maree MD et al (2002) "Effects of axial ligands on the photophysical properties of silicon octaphenoxyphthalocyanine." J Porphyr Phthalocyan 6 06: 373–376
Fery-Forgues S, Lavabre D (1999) "Are fluorescence quantum yields so tricky to measure? A demonstration using familiar stationery products." J Chem Educ 76 9: 1260
El-Sedik M et al (2012) "Synthesis, absorption and fluorescence properties of N-triazinyl derivatives of 2-aminoanthracene."Dyes Pigments 92 3: 1126–1131
Würth C et al (2012) "Determination of the absolute fluorescence quantum yield of rhodamine 6G with optical and photoacoustic methods–Providing the basis for fluorescence quantum yield standards." Talanta 9 30–37
Murphy CB et al (2004) "Probing Förster and Dexter energy-transfer mechanisms in fluorescent conjugated polymer chemosensors." J Phys Chem B 108 5 1537–1543
Larsen RW et al (1999) "Ground‐and Excited‐State Characterization of an Electrostatic Complex between Tetrakis‐(4‐Sulfonatophenyl) porphyrin and 16‐Pyrimidinium Crown‐4."Photochem Photobiol 69 4: 429–434
Micho J et al (2010) "Determinations of uranium (VI) binding properties with some metalloproteins (transferrin, albumin, metallothionein and ferritin) by fluorescence quenching."J Fluorescen 20 2: 581–590
Hanwell MD, Curtis DE, Lonie DC et al (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform 4:17. https://doi.org/10.1186/1758-2946-4-17
Neese F (2012) The ORCA program system, Wiley Interdiscip. Rev.: Comput. Mol Sci 2:73–78
Acknowledgements
The authors gratefully acknowledge COENFDD Centre, Rajkot, (O2H) –Ahmedabad, Gujarat University for providing instrumental Facilities, and Information and Library Network for ejournals.
Funding
The research was supported by Gujarat University-Ahmedabad India.
Author information
Authors and Affiliations
Contributions
Patrick F. Fernandes: (Supervision, investigation designed and directed the study, planned and carried out the experiments, wrote the manuscript). Dr. Divya R. Mishra: (Supervision, designed and directed the study, contributed to the interpretation of the results and characterized of the molecules). The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
For this type of study, the ethical approval was not required, because this study does not involve cell or animal manipulation.
Consent to Participate
All authors gave their consent to participate in the research.
Consent for Publication
All authors gave their consent to participate in the publication of the research.
Conflicts of Interest
The authors declare that there is no conflict of interests regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fernandes, P.F., Mishra, D.R. A Sensor for the Detection of Cr (III) and Fe (III) Ions Based on “Turn Off” Mechanism of Fluorescence with Computational Studies. J Fluoresc 32, 215–226 (2022). https://doi.org/10.1007/s10895-021-02787-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-021-02787-0