Abstract
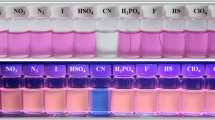
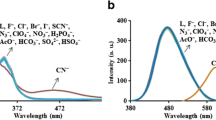
Cyanine-based probe-possessing indolium iodide and indole unit were synthesized in two-step with easy available raw material: a potential probe for the cyanide ion detection. The detecting ability of the probe was investigated and confirmed by a visual and instrumental approach. A noticeable color change from orange to colorless obtained only for cyanide ions and other added ions does not impart any changes visually and through UV and Fluorescence technique. To confirm the mechanism of sensing 1H-NMR recorded. From the result, the peak belonging to N-methyl displayed an upfield shift from 4.01 δ ppm to 2.74 δ ppm due to the disappearance of indolium iodide ion and the olefin protons peaks were shifted from 7.19 to 6.17 and 8.70 to 7.20 δ ppm confirms the nucleophilic addition of cyanide ion to the probe. Test kit from filter paper prepared for the real-time monitoring cyanide ion. The prepared strip is effective in detecting cyanide ion with a visual color change.










Similar content being viewed by others
Data Availability
All data available.
References
Schwartz B, Drueckhammer DG (1995) A simple method for determining the relative strengths of normal and low-barrier hydrogen bonds in solution: implications to enzyme catalysis. J Am Chem Soc 117:11902–11905
Clark JF, Clark DL, Whitener GD, Schroeder NC, Straus SH (1996) Isolation of soluble 99Tc as a compact solid using a recyclable, redox-active, metal-complex extractant. Environ Sci Technol 30:3124–3126
Sadyrbaeva TZ (2012) Gold (III) recovery from non-toxic electrolytes using hybrid electrodialysis–electrolysis process. Sep Purif Technol 86:262–265
Grossmann K (2010) Auxin herbicides: current status of mechanism and mode of action. Pest Manag Sci 66:113–120
Boening DW, Chew CM (1999) A critical review: general toxicity and environmental fate of three aqueous cyanide ions and associated ligands. Water Air Soil Pollut 109:67–79
Baskin SI, Brewer TG (1997) Medical aspects of chemical and biological warfare, ed. F. Sidell, E.T. Takafuji, D.R. Franz, TMM Publication, Washington, DC. 10: 271–286
Young C, Tidwell L, Anderson C (2001) Cyanide: social, industrial, and economic aspects; minerals, metals, and materials society: Warrendale, PA. 35
Guidelines for Drinking-Water Quality (2009) Cyanide in drinking water. World Health Organization, Geneva, Switzerland
Shan D, Mousty C, Cosnier S (2004) Subnanomolar cyanide detection at polyphenol oxidase/clay biosensors. Anal Chem 76:178–183
Safavi A, Maleki N, Shahbaazi HR (2004) Indirect determination of cyanide ion and hydrogen cyanide by adsorptive strip** voltammetry at a mercury electrode. Anal Chim Acta 503:213–231
Tian Y, Dasgupta PK, Mahon SB, Ma J, Brenner M, Wang J, Boss GR (2013) A disposable blood cyanide sensor. Anal Chim Acta 768:129–135
Christison TT, Rohrer JS (2007) Direct determination of free cyanide in drinking water by ion chromatography with pulsed amperometric detection. J Chromatogr A 1155:31–39
Xu Z, Chen X, Kim HN, Yoon J (2010) Sensors for the optical detection of cyanide ion. Chem Soc Rev 39:127–137
Jackson R, Oda RP, Bhandari RK, Mahon SB, Brenner M, Rockwood GA, Logue BA (2014) Development of a fluorescence-based sensor for rapid diagnosis of cyanide exposure. Anal Chem 86:1845–1852
Hong SJ, Yoo J, Kim SH, Kim JS, Yoon J, Lee CH (2009) Lee, beta-vinyl substituted calix 4 pyrrole as a selective ratiometric sensor for cyanide anion. Chem Commun 45:189–191
Chen CL, Chen YH, Chen CY, Sun SS (2006) Dipyrrole carboxamide derived selective ratiometric probes for cyanide ion. Org Lett 8:5053–5056
Yu HB, Zhao Q, Jiang ZX, Qin JG, Li Z (2010) A ratiometric fluorescent probe for cyanide: convenient synthesis and the proposed mechanism. Sens Actuators B 148:110–116
Lin Q, Lu TT, Zhu X, Sun B, Yang QP, Wei TB, Zhang YM (2015) A novel supramolecular metallogel-based high-resolution anion sensor array. Chem Commun 51:1635–1638
Shi BB, Zhang P, Wei TB, Yao H, Lin Q, Zhang YM (2013) Highly selective fluorescent sensing for CN− in water: utilization of the supramolecular self-assembly. Chem Commun 49:7812–7814
Lin Q, Sun B, Yang QP, Fu YP, Zhu X, Wei TB, Zhang YM (2014) Double metal ions competitively control the guest-sensing process: a facile approach to stimuli-responsive supramolecular gels. Chem Eur J 20:11457–11462
Yang L, Li X, Yang J, Qu Y, Hua J (2013) Colorimetric and ratiometric near-infrared fluorescent cyanide chemodosimeter based on phenazine derivatives. ACS Appl Mater Interfaces 5:1317–1326
Wang Y, Kim SH (2014) Colorimetric chemodosimeter for cyanide detection based on spiropyran derivative and its thermodynamic studies. Dyes Pigm 102:228–233
Li JJ, Wei W, Qi XL, Xu X, Liu YC, Lin QH, Dong W (2016) Rational design, synthesis of reaction-based dual-channel cyanide sensor in aqueous solution. Spectrochim Acta A 152:288–293
You GR, Park GJ, Lee SA, Choi YW, Kim YS, Lee JJ, Kim C (2014) A single chemosensor for multiple target anions: The simultaneous detection of CN- and OAc- in aqueous media. Sens Actuators B 202:645–655
El-Shishtawy RM, Al-Zahrani FAM, Al-amshany ZM, Asiri AM (2017) Synthesis of a new fluorescent cyanide chemosensor based on phenothiazine derivative. Sens Actuators B 240:288–296
Nandi LG, Nicoleti CR, Bellettini IC, Machado VG (2014) Optical chemosensor for the detection of cyanide in water based on ethyl(hydroxyethyl)cellulose functionalized with brooker’s merocyanine. Anal Chem 86:4653–4656
Cheng X, Zhou Y, Qin J, Li Z (2012) Reaction-based colorimetric cyanide chemosensors: rapid naked-eye detection and high selectivity. ACS Appl Mater Interfaces 4:2133–2138
Hu JW, Lin WC, Hsiao SY, Wu YH, Chen HW, Chen KY (2016) An indanedione-based chemodosimeter for selective naked-eye and fluorogenic detection of cyanide. Sens Actuators B 233:510–519
Lv X, Liu J, Liu Y, Zhao Y, Sun Y-Q, Wang P, Guo W (2011) Ratiometric fluorescence detection of cyanide based on a hybrid coumarin-hemicyanine dye: the large emission shift and the high selectivity. Chem Commun 47:12843–12845
Li J, Wei W, Qi X, Zuo G, Fang J, Dong W (2016) Highly selective colorimetric/fluorometric dual-channel sensor for cyanide based on ICT off in aqueous solution. Sens Actuators B 228:330–334
Acknowledgements
The authors thank Acharya Nagarjuna University for providing lab facilities to conduct research work.
Funding
No funding received from any source.
Author information
Authors and Affiliations
Contributions
All authors (Mahesh Gosi, Nagaraju Marepu, and Yeturua Sunandamma) made substantial contribution in preparing the manuscript.
Corresponding author
Ethics declarations
Consent to Participate
Informed consent obtained from all individual participants included in the study.
Competing Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gosi, M., Marepu, N. & Sunandamma, Y. Cyanine-based Fluorescent Probe for Cyanide Ion Detection. J Fluoresc 31, 1409–1415 (2021). https://doi.org/10.1007/s10895-021-02771-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-021-02771-8