Abstract
Purpose
We established a stable rat model of liver transplantation using Sprague-Dawley rats and Wistar rats in order to investigate the role of the IDO gene in acute rejection after rat liver transplantation.
Methods
IDO gene expression and IDO enzyme activity were quantified in liver syngeneic grafts and allografts using microdialysis-HPLC. Liver allografts were evaluated for IDO expression by histopathology. We measured liver function-related biomarkers in liver allografts which were re-infused with untreated or IFN-γ-treated dendritic cells (DCs).
Results
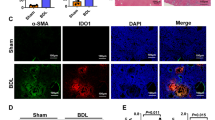
We found a significant increase in IDO gene expression and IDO enzyme activity in liver allografts compared the sham and syngeneic graft groups. There was a significant correlation between the number of IDO-positive cells and severity of acute rejection. IDO gene expression and enzyme activity was upregulated in the IFN-γ-treated DC group within 7 days after transplantation compared to the untreated DC group and survival rates were significantly improved.
Conclusions
Our results suggested that IDO gene expression correlates with the severity of acute rejection and that IFN-γ-induced IDO-positive DCs may attenuate acute rejection and catalyze local tryptophan metabolism via IDO enzyme expression, leading to immune tolerance after liver transplantation.









Similar content being viewed by others
References
Starzl TE, Marchioro TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659–76.
Seetharam A, Tiriveedhi V, Mohanakumar T. Alloimmunity and autoimmunity in chronic rejection. Curr Opin Organ Transplant. 2010;15(4):531–6. doi:10.1097/MOT.0b013e32833b31f4.
Lombardi G, Sagoo P, Scotta C, Fazekasova H, Smyth L, Tsang J, et al. Cell therapy to promote transplantation tolerance: a winning strategy? Immunotherapy. 2011;3(4 Suppl):28–31. doi:10.2217/imt.11.42.
Dresske B, Lin X, Huang DS, Zhou X, Fandrich F. Spontaneous tolerance: experience with the rat liver transplant model. Hum Immunol. 2002;63(10):853–61.
Blaha P, Bigenzahn S, Koporc Z, Sykes M, Muehlbacher F, Wekerle T. Short-term immunosuppression facilitates induction of mixed chimerism and tolerance after bone marrow transplantation without cytoreductive conditioning. Transplantation. 2005;80(2):237–43.
Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–61. doi:10.1126/science.1079490.
Taflin C, Nochy D, Hill G, Frouget T, Rioux N, Verine J, et al. Regulatory T cells in kidney allograft infiltrates correlate with initial inflammation and graft function. Transplantation. 2010;89(2):194–9. doi:10.1097/TP.0b013e3181c3ca11.
Wei S, Li J, Lian Z, Chen Y, Liu Z, You H, et al. Expression of glucocorticoid-induced tumor necrosis factor receptor ligand in rat graft after liver transplantation. Transplant Proc. 2011;43(5):1971–5. doi:10.1016/j.transproceed.2011.03.054.
Huang YL, Wang YZ, Chen JB, Wang F, Kang XP, **a JJ, et al. Prevention of acute and chronic allograft rejection by combinations of tolerogenic dendritic cells. Scand J Immunol. 2011;73(2):91–101. doi:10.1111/j.1365-3083.2010.02485.x.
van Kooten C, Lombardi G, Gelderman KA, Sagoo P, Buckland M, Lechler R, et al. Dendritic cells as a tool to induce transplantation tolerance: obstacles and opportunities. Transplantation. 2011;91(1):2–7.
Ezzelarab M, Thomson AW. Tolerogenic dendritic cells and their role in transplantation. Semin Immunol. 2011. doi:10.1016/j.smim.2011.06.007.
Natarajan S, Thomson AW. Tolerogenic dendritic cells and myeloid-derived suppressor cells: potential for regulation and therapy of liver auto- and alloimmunity. Immunobiology. 2010;215(9–10):698–703. doi:10.1016/j.imbio.2010.05.024.
Li J, Meinhardt A, Roehrich ME, Golshayan D, Dudler J, Pagnotta M, et al. Indoleamine 2,3-dioxygenase gene transfer prolongs cardiac allograft survival. Am J Physiol Heart Circ Physiol. 2007;293(6):H3415–23. doi:10.1152/ajpheart.00532.2007.
Laurence JM, Wang C, Zheng M, Cunningham S, Earl J, Tay SS, et al. Overexpression of indoleamine dioxygenase in rat liver allografts using a high-efficiency adeno-associated virus vector does not prevent acute rejection. Liver Transpl. 2009;15(2):233–41. doi:10.1002/lt.21662.
Medzhitov R, Shevach EM, Trinchieri G, Mellor AL, Munn DH, Gordon S, et al. Highlights of 10 years of immunology in Nature Reviews Immunology. Nat Rev Immunol. 2011;11(10):693–702. doi:10.1038/nri3063.
Terness P, Chuang JJ, Bauer T, Jiga L, Opelz G. Regulation of human auto- and alloreactive T cells by indoleamine 2,3-dioxygenase (IDO)-producing dendritic cells: too much ado about IDO? Blood. 2005;105(6):2480–6. doi:10.1182/blood-2004-06-2103.
Shumakov VI, Moisiuk Ia G, Shagidulin M. [The evolution of surgical techniques for donor liver procurement]. Vestn Ross Akad Med Nauk. 2006(12):7-11.
Cannazza G, Baraldi M, Braghiroli D, Tait A, Parenti C. High-performance liquid chromatographic method for the quantification of anthranilic and 3-hydroxyanthranilic acid in rat brain dialysate. J Pharm Biomed Anal. 2003;32(2):287–93.
Banff schema for grading liver allograft rejection: An international consensus document. Hepatology. 1997;25:6.
Yu G, Fang M, Gong M, Liu L, Zhong J, Feng W, et al. Steady state dendritic cells with forced IDO expression induce skin allograft tolerance by upregulation of regulatory T cells. Transpl Immunol. 2008;18(3):208–19. doi:10.1016/j.trim.2007.08.006.
Ingelsten M, Gustafsson K, Oltean M, Karlsson-Parra A, Olausson M, Haraldsson B, et al. Is indoleamine 2,3-dioxygenase important for graft acceptance in highly sensitized patients after combined auxiliary liver-kidney transplantation? Transplantation. 2009;88(7):911–9. doi:10.1097/TP.0b013e3181b72e49.
Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196(4):447–57.
Ge W, Jiang J, Arp J, Liu W, Garcia B, Wang H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation. 2010;90(12):1312–20. doi:10.1097/TP.0b013e3181fed001.
Palafox D, Llorente L, Alberu J, Torres-Machorro A, Camorlinga N, Rodriguez C, et al. The role of indoleamine 2,3 dioxygenase in the induction of immune tolerance in organ transplantation. Transplant Rev (Orlando). 2010;24(3):160–5. doi:10.1016/j.trre.2010.04.003.
Ghahary A, Li Y, Tredget EE, Kilani RT, Iwashina T, Karami A, et al. Expression of indoleamine 2,3-dioxygenase in dermal fibroblasts functions as a local immunosuppressive factor. J Invest Dermatol. 2004;122(4):953–64. doi:10.1111/j.0022-202X.2004.22409.x.
Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297(5588):1867–70. doi:10.1126/science.1073514.
Acknowledgements
None.
Funding
This study was supported by the National Nature Science Foundation of China (NSFC 30972952 and NSFC 81170445).
Author information
Authors and Affiliations
Corresponding authors
Additional information
**ng Sun and Zi-jun Gong contributed equally.
Rights and permissions
About this article
Cite this article
Sun, X., Gong, Zj., Wang, Zw. et al. IDO-Competent-DCs Induced by IFN-γ Attenuate Acute Rejection in rat Liver Transplantation. J Clin Immunol 32, 837–847 (2012). https://doi.org/10.1007/s10875-012-9681-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-012-9681-4