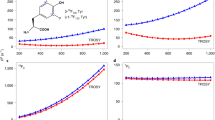
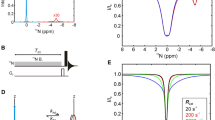
Abstract
Solution NMR spectroscopy is a particularly powerful technique for characterizing the functional dynamics of biomolecules, which is typically achieved through the quantitative characterization of chemical exchange processes via the measurement of spin relaxation rates. In addition to the conventional nuclei such as 15N and 13C, which are abundant in biomolecules, fluorine-19 (19F) has recently garnered attention and is being widely used as a site-specific spin probe. While 19F offers the advantages of high sensitivity and low background, it can be susceptible to artifacts in quantitative relaxation analyses due to a multitude of dipolar and scalar coupling interactions with nearby 1H spins. In this study, we focused on the ribose 2′-19F spin probe in nucleic acids and investigated the effects of 1H-19F spin interactions on the quantitative characterization of slow exchange processes on the millisecond time scale. We demonstrated that the 1H-19F dipolar coupling can significantly affect the interpretation of 19F chemical exchange saturation transfer (CEST) experiments when 1H decoupling is applied, while the 1H-19F interactions have a lesser impact on Carr-Purcell-Meiboom-Gill relaxation dispersion applications. We also proposed a modified CEST scheme to alleviate these artifacts along with experimental verifications on self-complementary RNA systems. The theoretical framework presented in this study can be widely applied to various 19F spin systems where 1H-19F interactions are operative, further expanding the utility of 19F relaxation-based NMR experiments.






Similar content being viewed by others
Code availability
The Python scripts and the Bruker-format pulse programs used in this study are available on https://github.com/YukiToyama/19F_CEST.
References
Abragam A (1961) The principles of nuclear magnetism. Clarendon Press, Oxford
Alderson TR, Kay LE (2021) NMR spectroscopy captures the essential role of dynamics in regulating biomolecular function. Cell 184:577–595. https://doi.org/10.1016/j.cell.2020.12.034
Altona C, Sundaralingam M (1973) Conformational analysis of the sugar ring in nucleosides and nucleotides. Improved method for the interpretation of proton magnetic resonance coupling constants. J Am Chem Soc 95:2333–2344. https://doi.org/10.1021/ja00788a038
Arntson KE, Pomerantz WCK (2016) Protein-observed fluorine NMR: a bioorthogonal approach for small molecule discovery. J Med Chem 59:5158–5171. https://doi.org/10.1021/acs.jmedchem.5b01447
Boeszoermenyi A, Ogórek B, Jain A et al (2020) The precious fluorine on the ring: fluorine NMR for biological systems. J Biomol NMR 74:365–379. https://doi.org/10.1007/s10858-020-00331-z
Bouvignies G, Vallurupalli P, Kay LE (2014) Visualizing side chains of invisible protein conformers by solution NMR. J Mol Biol 426:763–774. https://doi.org/10.1016/j.jmb.2013.10.041
Buchholz CR, Pomerantz WCK (2021) 19F NMR viewed through two different lenses: ligand-observed and protein-observed 19F NMR applications for fragment-based drug discovery. RSC Chem Biol 2:1312–1330. https://doi.org/10.1039/d1cb00085c
Cavanagh J, Skelton NJ, Fairbrother WJ et al (2010) Protein NMR spectroscopy: principles and practice. Elsevier, Amsterdam
Dalvit C, Vulpetti A (2019) Ligand-based fluorine NMR screening: principles and applications in drug discovery projects. J Med Chem 62:2218–2244. https://doi.org/10.1021/acs.jmedchem.8b01210
Danielson MA, Falke JJ (1996) Use of 19F NMR to probe protein structure and conformational changes. Annu Rev Biophys Biomol Struct 25:163–195. https://doi.org/10.1146/annurev.bb.25.060196.001115
Delaglio F, Grzesiek S, Vuister GW et al (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293. https://doi.org/10.1007/BF00197809
Ernst RR, Bodenhausen G, Wokaun A (1987) Principles of nuclear magnetic resonance in one and two dimensions. Clarendon Press, Oxford
Farrow NA, Zhang O, Forman-Kay JD, Kay LE (1994) A heteronuclear correlation experiment for simultaneous determination of 15N longitudinal decay and chemical exchange rates of systems in slow equilibrium. J Biomol NMR 4:727–734. https://doi.org/10.1007/BF00404280
Feeney J, McCormick JE, Bauer CJ et al (1996) 19F nuclear magnetic resonance chemical shifts of fluorine containing aliphatic amino acids in proteins: Studies on lactobacillus casei dihydrofolate reductase containing (2S,4S)-5-fluoroleucine. J Am Chem Soc 118:8700–8706. https://doi.org/10.1021/ja960465i
Freeman R, Hill HDW (1971) Fourier transform study of NMR spin–lattice relaxation by “progressive saturation.” J Chem Phys 54:3367
Frieden C, Hoeltzli SD, Bann JG (2004) The preparation of 19F-labeled proteins for NMR studies. Methods Enzymol 380:400–415. https://doi.org/10.1016/S0076-6879(04)80018-1
Gao J, Liang E, Ma R et al (2017) Fluorine pseudocontact shifts used for characterizing the protein-ligand interaction mode in the limit of NMR intermediate exchange. Angew Chem Int Ed Engl 56:12982–12986. https://doi.org/10.1002/anie.201707114
García De La Torre J, Huertas ML, Carrasco B (2000) Calculation of hydrodynamic properties of globular proteins from their atomic-level structure. Biophys J 78:719–730. https://doi.org/10.1016/S0006-3495(00)76630-6
Goldman M (1984) Interference effects in the relaxation of a pair of unlike spin-12 nuclei. J Magn Reson 60:437–452. https://doi.org/10.1016/0022-2364(84)90055-6
Gronenborn AM (2022) Small, but powerful and attractive: 19F in biomolecular NMR. Structure 30:6–14. https://doi.org/10.1016/j.str.2021.09.009
Guenneugues M, Berthault P, Desvaux H (1999) A method for determining B1 field inhomogeneity. Are the biases assumed in heteronuclear relaxation experiments usually underestimated? J Magn Reson 136:118–126. https://doi.org/10.1006/jmre.1998.1590
Guschlbauer W, Jankowski K (1980) Nucleoside conformation is determined by the electronegativity of the sugar substituent. Nucleic Acids Res 8:1421–1433. https://doi.org/10.1093/nar/8.6.1421
Hansen AL, Lundström P, Velyvis A, Kay LE (2012) Quantifying millisecond exchange dynamics in proteins by CPMG relaxation dispersion NMR using side-chain 1H probes. J Am Chem Soc 134:3178–3189. https://doi.org/10.1021/ja210711v
Hansen DF, Vallurupalli P, Kay LE (2008) An improved 15N relaxation dispersion experiment for the measurement of millisecond time-scale dynamics in proteins. J Phys Chem B 112:5898–5904. https://doi.org/10.1021/jp074793o
Helgstrand M, Härd T, Allard P (2000) Simulations of NMR pulse sequences during equilibrium and non-equilibrium chemical exchange. J Biomol NMR 18:49–63. https://doi.org/10.1023/A:1008309220156
Heller GT, Shukla VK, Figueiredo AM, Hansen DF (2024) Picosecond dynamics of a small molecule in its bound state with an intrinsically disordered protein. J Am Chem Soc 146:2319–2324. https://doi.org/10.1021/jacs.3c11614
Helmus JJ, Jaroniec CP (2013) Nmrglue: an open source Python package for the analysis of multidimensional NMR data. J Biomol NMR 55:355–367. https://doi.org/10.1007/s10858-013-9718-x
Henzler-Wildman K, Kern D (2007) Dynamic personalities of proteins. Nature 450:964–972. https://doi.org/10.1038/nature06522
Hull WE, Sykes BD (1974) Fluorotyrosine alkaline phosphatase. 19F nuclear magnetic resonance relaxation times and molecular motion of the individual fluorotyrosines. Biochemistry 13:3431–3437. https://doi.org/10.1021/bi00714a002
Hull WE, Sykes BD (1975a) Dipolar nuclear spin relaxation of 19F in multispin systems: application to 19F labeled proteins. J Chem Phys 63:867
Hull WE, Sykes BD (1975b) Fluorotyrosine alkaline phosphatase: Internal mobility of individual tyrosines and the role of chemical shift anisotropy as a 19F nuclear spin relaxation mechanism in proteins. J Mol Biol 98:121–153. https://doi.org/10.1016/S0022-2836(75)80105-7
Ishima R, Torchia DA (2003) Extending the range of amide proton relaxation dispersion experiments in proteins using a constant-time relaxation-compensated CPMG approach. J Biomol NMR 25:243–248. https://doi.org/10.1023/A:1022851228405
Ishima R, Wingfield PT, Stahl SJ et al (1998) Using amide 1H and 15N transverse relaxation to detect millisecond time-scale motions in perdeuterated proteins: application to HIV-1 protease. J Am Chem Soc 120:10534–10542. https://doi.org/10.1021/ja981546c
Jeener J, Meier BH, Bachmann P, Ernst RR (1979) Investigation of exchange processes by two-dimensional NMR spectroscopy. J Chem Phys 71:4546–4553. https://doi.org/10.1063/1.438208
Kalk A, Berendsen HJC (1976) Proton magnetic relaxation and spin diffusion in proteins. J Magn Reson 24:343–366. https://doi.org/10.1016/0022-2364(76)90115-3
Karplus M, McCammon JA (1983) Dynamics of proteins: elements and function. Annu Rev Biochem 52:263–300. https://doi.org/10.1146/annurev.bi.52.070183.001403
Kawasaki AM, Casper MD, Freier SM et al (1993) Uniformly modified 2’-deoxy-2’-fluoro phosphorothioate oligonucleotides as nuclease-resistant antisense compounds with high affinity and specificity for RNA targets. J Med Chem 36:831–841. https://doi.org/10.1021/jm00059a007
Kay LE, Nicholson LK, Delaglio F et al (1992) Pulse sequences for removal of the effects of cross correlation between dipolar and chemical-shift anisotropy relaxation mechanisms on the measurement of heteronuclear T1 and T2 values in proteins. J Magn Reson 97:359–375. https://doi.org/10.1016/0022-2364(92)90320-7
Kay LE, Torchia DA, Bax A (1989) Backbone dynamics of proteins as studied by 15N inverse detected heteronuclear NMR spectroscopy: application to staphylococcal nuclease. Biochemistry 28:8972–8979. https://doi.org/10.1021/bi00449a003
Kim TH, Mehrabi P, Ren Z et al (2017) The role of dimer asymmetry and protomer dynamics in enzyme catalysis. Science 355:2355. https://doi.org/10.1126/science.aag2355
Kitevski-LeBlanc JL, Prosser RS (2012) Current applications of 19F NMR to studies of protein structure and dynamics. Prog Nucl Magn Reson Spectrosc 62:1–33. https://doi.org/10.1016/j.pnmrs.2011.06.003
Krempl C, Sprangers R (2023) Assessing the applicability of 19F labeled tryptophan residues to quantify protein dynamics. J Biomol NMR 77:55–67. https://doi.org/10.1007/s10858-022-00411-2
Kreutz C, Kählig H, Konrat R, Micura R (2005) Ribose 2’-F labeling: a simple tool for the characterization of RNA secondary structure equilibria by 19F NMR spectroscopy. J Am Chem Soc 127:11558–11559. https://doi.org/10.1021/ja052844u
Levitt MH (1982) Symmetrical composite pulse sequences for NMR population inversion. II. Compensation of resonance offset. J Magn Reson 50:95–110. https://doi.org/10.1016/0022-2364(82)90035-X
Levitt MH, Di Bari L (1992) Steady state in magnetic resonance pulse experiments. Phys Rev Lett 69:3124–3127. https://doi.org/10.1103/PhysRevLett.69.3124
Liu JJ, Horst R, Katritch V et al (2012) Biased signaling pathways in β2-adrenergic receptor characterized by 19F-NMR. Science 335:1106–1110. https://doi.org/10.1126/science.1215802
Loria JP, Rance M, Palmer AG (1999) A relaxation-compensated Carr−Purcell−Meiboom−Gill sequence for characterizing chemical exchange by NMR spectroscopy. J Am Chem Soc 121:2331–2332. https://doi.org/10.1021/ja983961a
Lu M, Ishima R, Polenova T, Gronenborn AM (2019) 19F NMR relaxation studies of fluorosubstituted tryptophans. J Biomol NMR 73:401–409. https://doi.org/10.1007/s10858-019-00268-y
Luck LA, Vance JE, O’Connell TM, London RE (1996) 19F NMR relaxation studies on 5-fluorotryptophan- and tetradeutero-5-fluorotryptophan-labeled E. coli glucose/galactose receptor. J Biomol NMR 7:261–272. https://doi.org/10.1007/BF00200428
Lundström P, Hansen DF, Vallurupalli P, Kay LE (2009) Accurate measurement of alpha proton chemical shifts of excited protein states by relaxation dispersion NMR spectroscopy. J Am Chem Soc 131:1915–1926. https://doi.org/10.1021/ja807796a
Luy B, Barchi JJ, Marino JP (2001) S3E-E.COSY methods for the measurement of 19F associated scalar and dipolar coupling constants. J Magn Reson 152:179–184. https://doi.org/10.1006/jmre.2001.2386
Luy B, Marino JP (2001) Measurement and application of 1H–19F dipolar couplings in the structure determination of 2’-fluorolabeled RNA. J Biomol NMR 20:39–47. https://doi.org/10.1023/a:1011210307947
Manglik A, Kim TH, Masureel M et al (2015) Structural insights into the dynamic process of β2-adrenergic receptor signaling. Cell 161:1101–1111. https://doi.org/10.1016/j.cell.2015.04.043
Manoharan M, Akinc A, Pandey RK et al (2011) Unique gene-silencing and structural properties of 2’-fluoro-modified siRNAs. Angew Chem Int Ed Engl 50:2284–2288. https://doi.org/10.1002/anie.201006519
Millet O, Muhandiram DR, Skrynnikov NR, Kay LE (2002) Deuterium spin probes of side-chain dynamics in proteins. 1. Measurement of five relaxation rates per deuteron in (13)C-labeled and fractionally (2)H-enriched proteins in solution. J Am Chem Soc 124:6439–6448. https://doi.org/10.1021/ja012497y
Muhandiram DR, Yamazaki T, Sykes BD (1995) Measurement of 2H T1 and T1 rho: relaxation times in uniformly 13C-labeled and fractionally 2H-labeled proteins in solution. J Am Chem Soc. https://doi.org/10.1021/ja00151a018
Mulder FAA, Hon B, Mittermaier A et al (2002) Slow internal dynamics in proteins: application of NMR relaxation dispersion spectroscopy to methyl groups in a cavity mutant of T4 lysozyme. J Am Chem Soc 124:1443–1451. https://doi.org/10.1021/ja0119806
Nicholson LK, Kay LE, Baldisseri DM et al (1992) Dynamics of methyl groups in proteins as studied by proton-detected 13C NMR spectroscopy. Application to the leucine residues of staphylococcal nuclease. Biochemistry 31:5253–5263. https://doi.org/10.1021/bi00138a003
Ortega A, Amorós D, García de la Torre J (2011) Prediction of hydrodynamic and other solution properties of rigid proteins from atomic- and residue-level models. Biophys J 101:892–898. https://doi.org/10.1016/j.bpj.2011.06.046
Overbeck JH, Kremer W, Sprangers R (2020) A suite of 19F based relaxation dispersion experiments to assess biomolecular motions. J Biomol NMR 74:753–766. https://doi.org/10.1007/s10858-020-00348-4
Overbeck JH, Vögele J, Nussbaumer F et al (2023) Multi-site conformational exchange in the synthetic neomycin-sensing riboswitch studied by 19 F NMR. Angew Chem Int Ed Engl 62:e202218064. https://doi.org/10.1002/anie.202218064
Pallan PS, Greene EM, Jicman PA et al (2011) Unexpected origins of the enhanced pairing affinity of 2’-fluoro-modified RNA. Nucleic Acids Res 39:3482–3495. https://doi.org/10.1093/nar/gkq1270
Palmer AG 3rd (2004) NMR characterization of the dynamics of biomacromolecules. Chem Rev 104:3623–3640. https://doi.org/10.1021/cr030413t
Palmer AG 3rd, Kroenke CD, Loria JP (2001) Nuclear magnetic resonance methods for quantifying microsecond-to-millisecond motions in biological macromolecules. Methods Enzymol 339:204–238. https://doi.org/10.1016/s0076-6879(01)39315-1
Palmer AG, Skelton NJ, Chazin WJ et al (1992) Suppression of the effects of cross-correlation between dipolar and anisotropic chemical shift relaxation mechanisms in the measurement of spin-spin relaxation rates. Mol Phys 75:699–711. https://doi.org/10.1080/00268979200100511
Peng JW (2001) Cross-correlated (19)F relaxation measurements for the study of fluorinated ligand-receptor interactions. J Magn Reson 153:32–47. https://doi.org/10.1006/jmre.2001.2422
Picard L-P, Prosser RS (2021) Advances in the study of GPCRs by 19F NMR. Curr Opin Struct Biol 69:169–176. https://doi.org/10.1016/j.sbi.2021.05.001
Podbevsek P, Allerson CR, Bhat B, Plavec J (2010) Solution-state structure of a fully alternately 2′-F/2′-OMe modified 42-nt dimeric siRNA construct. Nucleic Acids Res 38:7298–7307. https://doi.org/10.1093/nar/gkq621
Popenda L, Adamiak RW, Gdaniec Z (2008) Bulged adenosine influence on the RNA duplex conformation in solution. Biochemistry 47:5059–5067. https://doi.org/10.1021/bi7024904
Scott LG, Hennig M (2016) Chapter three—19F-Site-specific-labeled nucleotides for nucleic acid structural analysis by NMR. In: Kelman Z (ed) Methods in enzymology. Academic Press, pp 59–87
Sekhar A, Kay LE (2019) An NMR view of protein dynamics in health and disease. Annu Rev Biophys 48:297–319. https://doi.org/10.1146/annurev-biophys-052118-115647
Sekhar A, Rosenzweig R, Bouvignies G, Kay LE (2016) Hsp70 biases the folding pathways of client proteins. Proc Natl Acad Sci USA 113:E2794–E2801. https://doi.org/10.1073/pnas.1601846113
Shaka AJ, Barker PB, Freeman R (1985) Computer-optimized decoupling scheme for wideband applications and low-level operation. J Magn Reson 64:547–552. https://doi.org/10.1016/0022-2364(85)90122-2
Shinya S, Katahira R, Furuita K et al (2022) 19F chemical library and 19F-NMR for a weakly bound complex structure. RSC Med Chem 13:1100–1111. https://doi.org/10.1039/d2md00170e
Sklenář V, Torchia D, Bax A (1987) Measurement of carbon-13 longitudinal relaxation using 1H detection. J Magn Reson 73:375–379. https://doi.org/10.1016/0022-2364(87)90214-9
Skrynnikov NR, Millet O, Kay LE (2002) Deuterium spin probes of side-chain dynamics in proteins. 2. Spectral density map** and identification of nanosecond time-scale side-chain motions. J Am Chem Soc 124:6449–6460. https://doi.org/10.1021/ja012498q
Solomon I (1955) Relaxation processes in a system of two spins. Phys Rev 99:559–565. https://doi.org/10.1103/PhysRev.99.559
Sørensen OW, Eich GW, Levitt MH et al (1984) Product operator formalism for the description of NMR pulse experiments. Prog Nucl Magn Reson Spectrosc 16:163–192. https://doi.org/10.1016/0079-6565(84)80005-9
Tiwari VP, Toyama Y, De D et al (2021) The A39G FF domain folds on a volcano-shaped free energy surface via separate pathways. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.2115113118
Toyama Y, Rangadurai AK, Kay LE (2022) Measurement of 1Hα transverse relaxation rates in proteins: application to solvent PREs. J Biomol NMR 76:137–152. https://doi.org/10.1007/s10858-022-00401-4
Vallurupalli P, Bouvignies G, Kay LE (2012) Studying “invisible” excited protein states in slow exchange with a major state conformation. J Am Chem Soc 134:8148–8161. https://doi.org/10.1021/ja3001419
Vallurupalli P, Bouvignies G, Kay LE (2013) A computational study of the effects of (13) C-(13) C scalar couplings on (13) C CEST NMR spectra: towards studies on a uniformly (13) C-labeled protein. ChemBioChem 14:1709–1713. https://doi.org/10.1002/cbic.201300230
Vallurupalli P, Scott L, Williamson JR, Kay LE (2007) Strong coupling effects during X-pulse CPMG experiments recorded on heteronuclear ABX spin systems: artifacts and a simple solution. J Biomol NMR 38:41–46. https://doi.org/10.1007/s10858-006-9139-1
Vallurupalli P, Sekhar A, Yuwen T, Kay LE (2017) Probing conformational dynamics in biomolecules via chemical exchange saturation transfer: a primer. J Biomol NMR 67:243–271. https://doi.org/10.1007/s10858-017-0099-4
Wang F, Ramakrishna SK, Sun P, Fu R (2021) Triple-pulse excitation: an efficient way for suppressing background signals and eliminating radio-frequency acoustic ringing in direct polarization NMR experiments. J Magn Reson 332:107067. https://doi.org/10.1016/j.jmr.2021.107067
Yuwen T, Huang R, Kay LE (2017a) Probing slow timescale dynamics in proteins using methyl 1H CEST. J Biomol NMR 68:215–224. https://doi.org/10.1007/s10858-017-0121-x
Yuwen T, Kay LE (2019) Revisiting 1HN CPMG relaxation dispersion experiments: a simple modification can eliminate large artifacts. J Biomol NMR 73:641–650. https://doi.org/10.1007/s10858-019-00276-y
Yuwen T, Kay LE (2018) A new class of CEST experiment based on selecting different magnetization components at the start and end of the CEST relaxation element: an application to 1H CEST. J Biomol NMR 70:93–102. https://doi.org/10.1007/s10858-017-0161-2
Yuwen T, Kay LE (2017) Longitudinal relaxation optimized amide 1H-CEST experiments for studying slow chemical exchange processes in fully protonated proteins. J Biomol NMR 67:295–307. https://doi.org/10.1007/s10858-017-0104-y
Yuwen T, Sekhar A, Kay LE (2017b) Separating dipolar and chemical exchange magnetization transfer processes in 1 H-CEST. Angew Chem Int Ed Engl 56:6122–6125. https://doi.org/10.1002/anie.201610759
Zhao C, Devany M, Greenbaum NL (2014) Measurement of chemical exchange between RNA conformers by 19F NMR. Biochem Biophys Res Commun 453:692–695. https://doi.org/10.1016/j.bbrc.2014.09.075
Acknowledgements
The authors are grateful to Prof. Lewis E. Kay (University of Toronto) for critical reading of the manuscript and many useful suggestions. This work was supported by the Japan Agency for Medical Research and Development (AMED) under Grant Number JP21ae0121028 for I.S.
Funding
Funding was provided by Japan Agency for Medical Research and Development (Grant No. JP21ae0121028).
Author information
Authors and Affiliations
Contributions
YT concevied the project, prepared NMR samples, performed NMR experiments, carried out simulations, and analyzed the data. YT and IS wrote the manuscirpt.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Toyama, Y., Shimada, I. Quantitative analysis of the slow exchange process by 19F NMR in the presence of scalar and dipolar couplings: applications to the ribose 2′-19F probe in nucleic acids. J Biomol NMR (2024). https://doi.org/10.1007/s10858-024-00446-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10858-024-00446-7