Abstract
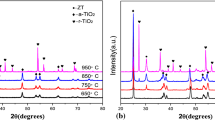
TiO2/Nb5+ film was prepared and deposited on 304 steel and preventive its potential for corrosion was evaluated. The film was obtained by the sol–gel method, and its deposition by the dip-coating process has been followed by thermal treatment. XRD results with Rietveld refinement revealed the occurrence of isomorphic substitution and predominance of anatase phase. Scanning electron microscopy confirmed the deposition of film on substrate. Corrosion properties of the TiO2/Nb5+ layer was determined by open-circuit potential, potentiodynamic polarization and electrochemical impedance spectroscopy (EIS). Polarization curves showed a corrosion current of 129.72 μA for 304 steel without coating, which was larger than that observed for the samples recovered with TiO2/Nb5+ films (9.00 μA). EIS revealed high charge transfer resistance in the molar ratio of 1 to 5% compared to the uncoated 304 steel, highlighting the protective effect of TiO2/Nb5+ films against corrosion.
Graphical abstract















Similar content being viewed by others
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations.
References
Bekker ACM, Verlinden JC (2018) Life cycle assessment of wire + arc additive manufacturing compared to green sand casting and CNC milling in stainless steel. J Clean Prod 177:438–447. https://doi.org/10.1016/j.jclepro.2017.12.148
Di Schino A (2020) Manufacturing and applications of stainless steels. Metals 10(3):327–329. https://doi.org/10.3390/met10030327
Banerjee MK (2017) 2.8 Heat treatment of commercial steels for engineering applications. Compr Mater Finish. https://doi.org/10.1016/B978-0-12-803581-8.09190-6
Atamert S, King JE (1991) Elemental partitioning and microstructural development in duplex stainless steel weld metal. Acta Metall Mater 39(3):273–285. https://doi.org/10.1016/0956-7151(91)90306-L
Ćurković L, Ćurković HO, Salopek S, Renjo MM, Šegota S (2013) Enhancement of corrosion protection of AISI 304 stainless steel by nanostructured sol–gel TiO2 films. Corros Sci 77:176–184. https://doi.org/10.1016/j.corsci.2013.07.045
Chasse KR, Raji S, Singh PM (2012) Effect of chloride ions on corrosion and stress corrosion cracking of duplex stainless steels in hot alkaline-sulfide solutions. Corrosion 68(10):932–949. https://doi.org/10.5006/0642
Tranchida G, Clesi M, di Franco F, di Quarto F, Santamaria M (2018) Electronic properties and corrosion resistance of passive films on austenitic and duplex stainless steels. Electrochim Acta 273:412–423. https://doi.org/10.1016/j.electacta.2018.04.058
Rosidah AA, Setyowati VA, Suheni S, Rijayanto R (2021) The effect of time variation on the steels corrosion rate in 0.5 M H2SO4 solution. J Mech Eng Sci Innov 1(2):49–55. https://doi.org/10.31284/j.jmesi.2021.v1i2.2183
Koch G (2017) Cost of corrosion. In: Trends in oil and gas corrosion research and technologies. Elsevier, New York, pp 3–30. https://doi.org/10.1016/B978-0-08-101105-8.00001-2
Hou B, Li X, Ma X, Du C, Zhang D, Zheng M, Xu W, Lu D, Ma F (2017) The cost of corrosion in China. Npj Mater Degrad. https://doi.org/10.1038/s41529-017-0005-2
Kumar SSA, Bashir S, Ramesh K, Ramesh S (2021) New perspectives on graphene/graphene oxide based polymer nanocomposites for corrosion applications: the relevance of the graphene/polymer barrier coatings. Prog Org Coat 154:106215–106222. https://doi.org/10.1016/j.porgcoat.2021.106215
Brella M, Taabouche A, Gharbi B, Gheriani R, Bouachiba Y, Bouabellou A, Serrar H, Touil S, Laggoune K, Boudissa M (2022) Comparison of thin films of titanium dioxide deposited by sputtering and sol–gel methods for waveguiding applications. Semiconductors 56(3):234–239. https://doi.org/10.1134/S106378262106004X
Zeribi F, Attaf A, Derbali A, Saidi H, Benmebrouk L, Aida MS, Dahnoun M, Nouadji R, Ezzaouia H (2022) Dependence of the physical properties of titanium dioxide (TiO2) thin films grown by sol–gel (spin-coating) process on thickness. ECS J Solid State Sci Technol 11(2):023003–023010. https://doi.org/10.1149/2162-8777/ac5168
Racovita AD (2022) Titanium dioxide: structure, impact, and toxicity. Int J Environ Res Pub Health 19(9):5681–5700. https://doi.org/10.3390/ijerph19095681
Gomes GHM, de Jesus MAML, Ferlauto AS, Viana MM, Mohallem NDS (2021) Characterization and application of niobium-doped titanium dioxide thin films prepared by sol–gel process. Appl Phys A 127(8):641–655. https://doi.org/10.1007/s00339-021-04781-6
Jana S, Debnath AK, Veerender P, Bahadur J, Kishor J, Chauhan AK, Bhattacharya D (2022) Investigation of thermally induced changes on structure, morphology and stoichiometry of sputter deposited titanium oxide thin films. Thin Solid Films 763:139608–139620. https://doi.org/10.1016/j.tsf.2022.139608
Wei B, Calatayud M (2022) Hydrogen activation on anatase TiO2: effect of surface termination. Catal Today 397–399:113–120. https://doi.org/10.1016/j.cattod.2021.11.020
Wu X, Liu Y (2023) Phase change and crystal growth of TiO2 in metatitanic acid. Ceram Int 49(3):4607–4613. https://doi.org/10.1016/j.ceramint.2022.09.346
Duan Y, Zhao G, Liu X, Ma J, Chen S, Song Y, Pi X, Yu X, Yang D, Zhang Y, Guo F (2021) Low-temperature processed tantalum/niobium co-doped TiO2 electron transport layer for high-performance planar perovskite solar cells. Nanotechnology 32(24):245201–245209. https://doi.org/10.1088/1361-6528/abeb37
Eraslan FS, Gecu R (2023) Chemical composition optimization of Al2O3–TiO2 composite coatings for enhanced wear and corrosion resistance. Surf Coat Technol 474:130053–130064. https://doi.org/10.1016/j.surfcoat.2023.130053
Gecu R, Birol B, Özcan M (2022) Improving wear and corrosion protection of AISI 304 stainless steel by Al2O3–TiO2 hybrid coating via sol–gel process. Trans IMF 100(6):324–332. https://doi.org/10.1080/00202967.2022.2117884
Joskowska D, Pomoni K, Vomvas A, Kościelska B, Anastassopoulos DL (2010) On electrical and photoconductive properties of mixed Nb2O5/TiO2 sol–gel thin films. J Non-Cryst Solids 356(37–40):2042–2048. https://doi.org/10.1016/j.jnoncrysol.2010.05.075
Santos BGP et al (2024) Development of TiO2/Nb2O5 films and evaluation of corrosion prevention in AISI 304. Steel Res Int. https://doi.org/10.1002/srin.202300423
Ferreira MOA, Gelamo RV, Marino CEB, da Silva BP, Aoki IV, da Luz MS, Alexopoulos ND, Leite NB, Moreto JA (2022) Effect of niobium oxide thin film on the long-term immersion corrosion of the 2198–T851 aluminum alloy. Materialia 22:101407–101415. https://doi.org/10.1016/j.mtla.2022.101407
Santos E, Catto AC, Peterline AF, Avansi W Jr (2022) Transition metal (Nb and W) doped TiO2 nanostructures: The role of metal do** in their photocatalytic activity and ozone gas-sensing performance. Appl Surf Sci 579:152146–152157. https://doi.org/10.1016/j.apsusc.2021.152146
Pehlivan E, Tepehan FZ, Tepehan GG (2005) Effect of TiO2 mixtures on the optical, structural and electrochromic properties of Nb2O5 thin films. Sol Energy Mater Sol Cells 87(1–4):317–322. https://doi.org/10.1016/j.solmat.2004.07.030
Kaur N et al (2020) Titanium dioxide: achievements in chemical sensing. Materials 13(13):2974–2995. https://doi.org/10.3390/ma13132974
Fleaca CT et al (2015) Laser oxidative pyrolysis synthesis and annealing of TiO2 nanoparticles embedded in carbon–silica shells/matrix. Appl Surf Sci 336:226–233. https://doi.org/10.1016/j.apsusc.2014.11.106
Read RT (1998) Book review: dielectric and mechanical relaxation in materials: analysis, interpretation and application to polymers. Polym Int 45(1):127–128
Nassar EJ, Ciuffi KJ, Gonçalves RR, Messaddeq Y, Ribeiro SJL (2003) Filmes de titânio-silício preparados por “spin” e “dip-coating.” Quim Nova 26(5):674–677. https://doi.org/10.1590/S0100-40422003000500010
Tekdir H, Yetim T, Yetim AF (2021) Corrosion properties of ceramic-based TiO2 films on plasma oxidized Ti6Al4V/316L Layered Implant Structured Manufactured by Selective Laser Melting. J Bionic Eng 18(4):944–957. https://doi.org/10.1007/s42235-021-0055-6
Yetim T et al (2022) Synthesis and characterization of wear and corrosion resistant Ni-doped Al2O3 nanocomposite ceramic coatings by sol–gel method. Surf Coat Technol 444:128659–128671
Abd E-L, Khalaf M (2015) Corrosion resistance of ZrO2–TiO2 nanocomposite multilayer thin films coated on carbon steel in hydrochloric acid solution. Mater Charact 108:29–41. https://doi.org/10.1016/j.matchar.2015.08.010
Eraslan FS, Gecu R (2024) Enhancing wear and corrosion resistance of TiO2–Al2O3 hybrid coatings by the development of TiO2–Al2O3 bilayer film coatings. Ceram Int. https://doi.org/10.1016/j.ceramint.2024.04.399
Khalaf MM, Abd El-Lateef HM (2016) Corrosion protection of mild steel by coating with TiO2 thin films co-doped with NiO and ZrO2 in acidic chloride environments. Mater Chem Phys 177:250–265. https://doi.org/10.1016/j.matchemphys.2016.04.026
Ribeiro DV, Souza CAC, Abrantes JCC (2015) Use of electrochemical impedance spectroscopy (EIS) to monitoring the corrosion of reinforced concrete. Revista IBRACON de Estruturas e Materiais 8(4):529–546. https://doi.org/10.1590/S1983-41952015000400007
Acknowledgements
The authors would like to thank the academic institution Centro Federal de Educação Tecnológica de Minas Gerais—CEFETMG and the CNPq, Grant Number: 407799/2022-2.
Funding
This work was supported by Grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (308278/2020–8) and the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (APQ-02052–21 and APQ-02506–22). Scholarships were provided by the Centro Federal de Educação Tecnológica de Minas Gerais—CEFETMG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dias, M.S., Santos, B.G.P., Machado, A.A. et al. Development, characterization and evaluation of TiO2/Nb5+ thin films with potential for corrosion prevention of AISI 304 steel in acid medium containing chloride ions. J Mater Sci (2024). https://doi.org/10.1007/s10853-024-09906-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10853-024-09906-9