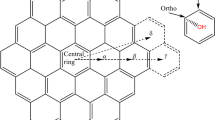
Abstract
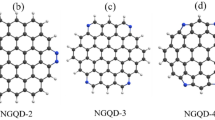
Graphene quantum dots (GQDs) with heteroatomic do** have emerged as a new type of fluorescent probing nanomaterials. The critical point of the application is the precise recognition of the controlling mechanism for varied fluorescent behavior. Herein, density functional theory simulation was performed to investigate the fluorescent behavior of p- and n-type heteroatomic edge-doped GQDs. The n-type (nitrogen)-doped GQDs generally have not shown an obvious fluorescent change with the Hg atom adsorption. However, the p-type (boron)-doped GQDs show a fluorescence red-shift beyond the visible light spectrum accompanied by a weak oscillator strength. This unique fluorescent quenching suggests that the p-type do** could probably have a probing function for elemental Hg. We also characterize the distinct fluorescent behavior for a series of GQDs with co-atomic pair do**. Natural transitional orbitals were applied to examine the energetic variation and electron–hole pair transition. The different photoluminescent phenomena could be ascribed to the differential orbital interactions between GQDs and Hg with varied charge transfers. Our simulation provides a novel insight into the fluorescent behavior of atomic-doped GQDs, which might be valuable for designing doped GQDs and exploring new optical probing applications.
Graphical Abstract









Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Sk MA, Ananthanarayanan A, Huang L, Lim KH, Chen P (2014) Revealing the tunable photoluminescence properties of graphene quantum dots. J Mater Chem C 2:6954–6960
Novoselov KS, Geim AK, Morozov SV et al (2005) Two-dimensional gas of massless dirac fermions in graphene. Nature 438:197–200
Florian L, Christoph S, Joachim B (2009) Graphene quantum dots: beyond a dirac billiard. Phys Rev B 79:115423
Lin LP, Rong MC, Luo F, Chen DM, Wang YR, Chen X (2014) Luminescent graphene quantum dots as new fluorescent materials for environmental and biological applications. Trends Anal Chem 54:83–102
Abbas A, Mariana LT, Phan AN (2018) Biomass-waste derived graphene quantum dots and their applications. Carbon 140:77–99
Li NN, Li RY, Li ZJ, Yang YQ, Wang GL, Gu ZG (2019) Pentaethylenehexamine and histidine-functionalized graphene quantum dots for ultrasensitive fluorescence detection of microRNA with target and molecular beacon double cycle amplification strategy. Sens Actuators, B Chem 283:666–676
Murugan N, Prakash M, Jayakumar M, Sundaramurthy A, Sundramoorthy AK (2019) Green synthesis of fluorescent carbon quantum dots from eleusine coracana and their application as a fluorescence ‘turn-off’ sensor probe for selective detection of Cu2+. Appl Surf Sci 476:468–480
Mukherjee D, Das P, Kundu S, Mandal B (2022) Engineering of graphene quantum dots by varying the properties of graphene oxide for fluorescence detection of picric acid. Chemosphere 300:134432
Demie K, Trevor PH, Che RS et al (2015) Electronic structure modification of ion implanted graphene: the spectroscopic signatures of p- and n-type do**. ACS Nano 9:11398–11407
Li HM, Lee D, Qu D, Liu X, Ryu J, Seabaugh A, Yoo WJ (2015) Ultimate thin vertical p-n junction composed of two-dimensional layered molybdenum disulfide. Nat Commun 6:6564
Yuan D, Liu W, Zhu X (2023) Efficient and air-stable n-type do** in organic semiconductors. Chem Soc Rev 52:3842–3872. https://doi.org/10.1039/d2cs01027e37183967
Wang S, Zuo G, Kim J, Sirringhaus H (2022) Progress of conjugated polymers as emerging thermoelectric materials. Prog Polym Sci 129:101548
Zhang Y, Zhao J, Sun H, Zhu Z, Zhang J, Liu Q (2018) B, N, S, Cl doped graphene quantum dots and their effects on gas-sensing properties of Ag-LaFeO3. Sens Actuators, B Chem 266:364–374
Morales Narvaez E, Arben M (2012) Graphene oxide as an optical biosensing platform. Adv Mater 24:3298–3308
Lakowicz JR (2006) Principles of fluorescence spectroscopy, 3n edn. Springer, New York, pp 27–61
Liu ZY, Mo ZL, Liu NJ, Guo RB, Niu XH, Zhao P, Yang X (2020) One-pot synthesis of highly fluorescent boron and nitrogen co-doped graphene quantum dots for the highly sensitive and selective detection of mercury ions in aqueous media. J Photochem Photobiol, A 389:112255
Yang Y, Zou T, Wang Z et al (2019) The fluorescent quenching mechanism of N and S co-Doped graphene quantum dots with Fe(3+) and Hg(2+) ions and their application as a novel fluorescent sensor. Nanomaterials 9(5):738
Yeh PC, Yoon S, Kurniawan D, Chung YG, Chiang WH (2021) Unraveling the fluorescence quenching of colloidal graphene quantum dots for selective metal ion detection. ACS Appl Nano Mater 4:5636–5642
Hanaoka K, Iwaki S, Yagi K et al (2022) General design strategy to precisely control the emission of fluorophores via a twisted intramolecular charge transfer (TICT) process. J Am Chem Soc 144:19778–19790
Feng J, Dong H, Pang B, Shao F, Zhang C, Yu L, Dong L (2018) Theoretical study on the optical and electronic properties of graphene quantum dots doped with heteroatoms. Phys Chem Chem Phys 20:15244–15252
Rani P, Dalal R, Srivastava S (2023) Effect of surface modification on optical and electronic properties of graphene quantum dots. Appl Surf Sci 609:155379
Zhang JJ, Ma J, Feng XL (2022) Precision synthesis of boron-doped graphene nanoribbons: recent progress and perspectives. Macromol Chem Phys 224:2200232
Chen C, Lu J, Lv Y et al (2022) Heteroatom-edged [4] triangulene: facile synthesis and two-dimensional on-surface self-assemblies. Angew Chem Int Ed 61:e202212594
Lee W, Bae GN (2009) Removal of elemental mercury (Hg(0)) by nanosized V2O5/TiO2 catalysts. Environ Sci Technol 43:1522–1527
Xu YF, Tang HJ, Liu LM, Hua M, Chen KX, Duan YF (2022) Hierarchical Fe/ZSM-5 zeolites for efficient Hg0 removal from coal-fired flue gas. Chem Eng J 450:138180
Boening DW (2000) Ecological effects, transport, and fate of mercury: a general review. Chemosphere 40:1335–1351
Kim KH, Kabir E, Jahan SA (2016) A review on the distribution of Hg in the environment and its human health impacts. J Hazard Mater 306:376–385
Brombach CC, Pichler T (2019) Determination of ultra-low volatile mercury concentrations in sulfur-rich gases and liquids. Talanta 199:277–284
Evan JG, Henry WP, Richard AH (2000) Novel sorbents for mercury removal from flue gas. Ind Eng Chem Res 39:1020–1029
He CL, Cheng GL, Zheng CB, Wu L, Lee Y, Hou XD (2015) Photochemical vapor generation and in situ preconcentration for determination of mercury by graphite furnace atomic absorption spectrometry. Anal Methods 7:3015–3021
Liu T, Liu J, Mao X, Huang X, Qian Y (2023) Novel platinum-nickel composite trap for simultaneous and direct determination of mercury and cadmium in soil and its mechanism study. Anal Chem 95:594–601
Gu H, Hao L, Ye H, Ma P, Wang Z (2021) Nuclease-assisted target recycling signal amplification strategy for graphene quantum dot-based fluorescent detection of marine biotoxins. Microchim Acta 188:1–9
Huang JT, Liu Y, Lai ZH, Hu J, Zhou F, Zhu JC (2022) Electronic structure and optical properties of non-metallic modified graphene: a first-principles study. Commun Theor Phys 74:035501
Sung K, Sung Won H, Min Kook K et al (2012) Anomalous behaviors of visible luminescence from graphene quantum dots: interplay between size and shape. ACS Nano 6:8203–8208
Te Velde G, Bickelhaupt FM, Baerends EJ, Fonseca Guerra C, Van Gisbergen SJA, Snijders JG, Ziegler T (2001) Chemistry with ADF. J Comput Chem 22:931–967
Devlin FJ, Stephens PJ, Cheeseman JR, Frisch MJ (1997) Ab initio prediction of vibrational absorption and circular dichroism spectra of chiral natural products using density functional theory: camphor and fenchone. J Phys Chem A 101:6322–6333
Grimme S, Ehrlich S, Goerigk L (2011) Effect of the dam** function in dispersion corrected density functional theory. J Comput Chem 32:1456–1465
Van Lenthe E, Baerends EJ (2003) Optimized slater-type basis sets for the elements 1–118. J Comput Chem 24:1142–1156
Van Lenthe E, Ehlers A, Baerends EJ (1999) Geometry optimizations in the zero order regular approximation for relativistic effects. J Chem Phys 110:8943–8953
Darvish Ganji M, Hosseini Khah SM, Amini Tabar Z (2015) Theoretical insight into hydrogen adsorption onto graphene: a first-principles B3LYP-D3 study. Phys Chem Chem Phys 17:2504–2511
Yuan K, Zhao RS, Zheng JJ et al (2017) Van der waals heterogeneous layer-layer carbon nanostructures involving pi···H-C-C-H···pi···H-C-C-H stacking based on graphene and graphane sheets. J Comput Chem 38:730–739
Miao SS, Xu ZJ, Yang XN (2020) DFT study of non-covalent interaction mechanisms of solvents with GO surfaces and the solvent-mediated GO interaction. Appl Surf Sci 499:143926
Lu YS, Huang LJ, Guo YN, Yang XN (2021) Theoretical insights into origin of graphene oxide acidity and relating behavior of oxygen-containing groups in water. Carbon 183:355–361
Kronik L, Stein T, Refaely Abramson S, Baer R (2012) Excitation gaps of finite-sized systems from optimally tuned range-separated hybrid functionals. J Chem Theory Comput 8:1515–1531
Cooper DR, D’anjou B, Ghattamaneni N et al (2012) Experimental review of graphene. ISRN Condens Matter Phys. https://doi.org/10.5402/2012/501686
So H, Martin HG (1999) Time-dependent density functional theory within the tamm-dancoff approximation. Chem Phys Lett 314:291–299
Weerawardene KL, Aikens CM (2016) Theoretical insights into the origin of photoluminescence of Au25(SR)18- nanoparticles. J Am Chem Soc 138:11202–11210
Meng Z, Yang X, Li H (2022) DFT-based theoretical simulation on electronic transition for graphene oxides in solvent media. J Mol Liq 348:118049
Bader RFW (1985) Atoms in molecules. Acc Chem Res 18:9–15
Mitoraj MP, Michalak A, Ziegler T (2009) On the nature of the agostic bond between metal centers and β-Hydrogen atoms in alkyl complexes. An analysis based on the extended transition state method and the natural orbitals for chemical valence scheme (ETS-NOCV). Organometallics 28:3727–3733
Etienne T (2015) Transition matrices and orbitals from reduced density matrix theory. J Chem Phys 142:244103
Li HL, Zu HX, Yang ZQ, Yang JP, Xu H, Qu WQ (2021) The adsorption mechanisms of Hg0 on marcasite-type metal selenides: the influences of metal-terminated site. Chem Eng J 406:126723
Sun SJ, Zhang DS, Li CY, Wang YJ, Yang QS (2014) Density functional theory study of mercury adsorption and oxidation on CuO(111) surface. Chem Eng J 258:128–135
Dai X, Zhou X, Liu H, Wang T, Zhang Y, Zhang H, Sun B (2021) Molecular-level insights into the immobilization of vapor-phase mercury on Fe/Co/Ni-doped hierarchical molybdenum selenide. J Hazard Mater 420:126583
Liu Z, Zhang YL, Wang BY, Cheng H, Cheng XR, Huang ZC (2018) DFT study on Al-doped defective graphene towards adsorption of elemental mercury. Appl Surf Sci 427:547–553
Li X, Cao X (2018) On the covalence in H2-AuX (X=F-I). Int J Hydrogen Energy 43:1709–1717
Su P, Li H (2009) Energy decomposition analysis of covalent bonds and intermolecular interactions. J Chem Phys 131:014102
Chen L, Guo Y, Xu Z, Yang X (2018) Multiscale simulation of the interaction and adsorption of ions on a hydrophobic graphene surface. ChemPhysChem 19:2954–2960
Guo J, Yang Y, Dou C, Wang Y (2021) Boron-containing organic diradicaloids: dynamically modulating singlet diradical character by lewis acid-base coordination. J Am Chem Soc 143:18272–18279
Ito M, Shirai S, **e Y et al (2022) Fluorescent organic pi-radicals stabilized with boron: featuring a SOMO-LUMO electronic transition. Angew Chem Int Ed 61:e202201965
Zia MA, Baig MA (2004) Two-step laser optogalvanic spectroscopy of the odd-parity rydberg states of atomic mercury. Eur Phys J D 28:323–330
Zhao ZY, Zhang XL, Song XD, Hao C (2021) Fluorescence quenching mechanism of 9-hydroxyphenal-1-one carbon quantum dots by Cu2+ ions: an experimental and computational investigation. J Photochem Photobiol A 408:113103
Englman R, Jortner J (1970) The energy gap law for radiationless transitions in large molecules. Mol Phys 18:145–164
Chen S, Ullah N, Zhao Y, Zhang R (2019) Nonradiative excited-state decay via conical intersection in graphene nanostructures. ChemPhysChem 20:2754–2758
Chen S, Ullah N, Wang T, Zhang R (2018) Tuning the optical properties of graphene quantum dots by selective oxidation: a theoretical perspective. J Mater Chem C 6:6875–6883
Feng JG, Dong HZ, Pang BL, Chen YJ, Yu LY, Dong LF (2019) Tuning the electronic and optical properties of graphene quantum dots by selective boronization. J Mater Chem C 7:237–246
Zhang Q, Zhou YL, Yu YB, Chen BY, Hong JM (2020) Exploring catalytic performance of boron-doped graphene electrode for electrochemical degradation of acetaminophen. Appl Surf Sci 508:145111
Mortazavi B, Javvaji B, Shojaei F, Rabczuk T, Shapeev AV, Zhuang X (2021) Exceptional piezoelectricity, high thermal conductivity and stiffness and promising photocatalysis in two-dimensional MoSi2N4 family confirmed by first-principles. Nano Energy 82:105716
Acknowledgements
This work was supported by the National Natural Science Foundation of China under Grants 21676136. The computational resources generously provided by High Performance Computing Center of Nan**g Tech University are greatly appreciated.
Funding
National Natural Science Foundation of China, 21676136, **aoning Yang
Author information
Authors and Affiliations
Contributions
ZM contributed to formal analysis, investigation, data curation, and writing—original draft. ZX contributed to formal analysis and investigation. XY contributed to conceptualization, project administration, investigation, methodology, and writing—original draft.
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts to declare.
Ethical approval
This paper does not involve experiments on ethical issues.
Additional information
Handling Editor: Yaroslava Yingling.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10853_2023_9114_MOESM1_ESM.docx
Supplementary Tables S1-S3 and Figs. S1-S13 include detailed composition and electron distribution of HOMO and LUMO in pristine GQDs, the fluorescent spectra, and NTOs patterns of GQDs with Hg adsorption. (DOCX 14193 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meng, Z., Xu, Z. & Yang, X. Theoretical investigation of fluorescent behavior for p- and n-type atomic-doped graphene quantum dots interacting with Hg (0). J Mater Sci 58, 17527–17542 (2023). https://doi.org/10.1007/s10853-023-09114-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-09114-x