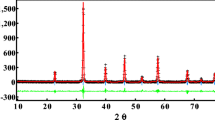
Abstract
Cation ordering/disordering features in perovskite-like oxides LnBaMn2O6−δ where Ln = Pr, Nd, Sm were studied by means of a combination of thermogravimetry and X-ray powder diffraction techniques in a wide range of temperatures. Variation in lanthanide size was shown to be an effective route for the shift in temperature of ordering and disordering. As a result, it was found coexistence of three different phases which can be obtained separately: disordered cubic Ln0.5Ba0.5MnO3–δ, and ordered kinds of LnBaMn2O6−δ having δ ≈ 0 or 1.0. The respective ambient conditions around stability boundaries for the whole studied phases were successfully determined. Additionally the main reasons for inducing ordering as well as disordering processes were clarified by the use of in-situ XRD and DSC methods. A combination of the experimentally obtained data with the results of limited studies reported in literature allows one to organize the available information about double manganites giving some explanation of ordering processes as well as appropriate conditions used to obtain oxides with desired ordering degree.












Similar content being viewed by others
Data availability
Data available on requirements.
References
Kudyakova VS, Politov BV, Suntsov AY, Kozhevnikov VL (2020) Phase stability and thermodynamic properties of PrBaMn2O6–δ. J Solid State Chem 287:6–11. https://doi.org/10.1016/j.jssc.2020.121382
Kudyakova VS, Politov BV, Chukin AV et al (2020) Phase stability and oxygen storage capacity of PrBaMn2O6–δ. Mater Lett 269:127650. https://doi.org/10.1016/j.matlet.2020.127650
Kudyakova VS, Shalamova AM, Chukin AV, Suntsov AY (2021) Enhanced thermal stability and red-ox activity of PrBaMn2−xFexO6−δ oxides. Mater Res Bull 140:111309. https://doi.org/10.1016/j.materresbull.2021.111309
Jeamjumnunja K, Gong W, Makarenko T, Jacobson AJ (2016) A determination of the oxygen non-stoichiometry of the oxygen storage materials LnBaMn2O5+δ (Ln=Gd, Pr). J Solid State Chem 239:36–45. https://doi.org/10.1016/j.jssc.2016.04.006
Jeamjumnunja K, Gong W, Makarenko T, Jacobson AJ (2015) A determination of the oxygen non-stoichiometry of the oxygen storage material YBaMn2O5+δ. J Solid State Chem 230:397–403. https://doi.org/10.1016/j.jssc.2015.07.044
Motohashi T, Kimura M, Inayoshi T et al (2015) Redox characteristics variations in the cation-ordered perovskite oxides BaLnMn2O5+δ (Ln = Y, Gd, Nd, and La) and Ca2Al1−xGaxMnO5+δ (0 ≤ x ≤ 1). Dalton Trans 44:10746–10752. https://doi.org/10.1039/c4dt03863k
Tonus F, Bahout M, Dorcet V et al (2016) Redox behavior of the SOFC electrode candidate NdBaMn2O5+δ investigated by high-temperature in-situ neutron diffraction: first characterisation in real time of an LnBaMn2O5.5 intermediate phase. J Mater Chem A 4:11635–11647. https://doi.org/10.1039/c6ta03224a
Liu Y, Wang Z, Veder JPM et al (2018) Highly defective layered double perovskite oxide for efficient energy storage via reversible pseudocapacitive oxygen-anion intercalation. Adv Energy Mater 8:1–11. https://doi.org/10.1002/aenm.201702604
Motohashi T, Ueda T, Masubuchi Y et al (2010) Remarkable oxygen intake release capability of BaYMn2O5+δ. Chem Mater 22:3192–3196. https://doi.org/10.1021/cm100290b
Garcia-Garcia FJ, Sayagués MJ, Gotor FJ (2021) A novel, simple and highly efficient route to obtain PrBaMn2O5+δ double perovskite: mechanochemical synthesis. Nanomaterials 11:1–18. https://doi.org/10.3390/nano11020380
Sengodan S, Ju YW, Kwon O et al (2017) Self-decorated MnO nanoparticles on double perovskite solid oxide fuel cell anode by in-situ exsolution. ACS Sustain Chem Eng 5:9207–9213. https://doi.org/10.1021/acssuschemeng.7b02156
Kim K, Joo S, Huang R et al (2021) Mechanistic insights into the phase transition and metal ex-solution phenomena of Pr0.5Ba0.5Mn0.85Co0.15O3−δ from simple to layered perovskite under reducing conditions and enhanced catalytic activity. Energy Environ Sci 14:873–882. https://doi.org/10.1039/d0ee02875d
Chen M, Xu X, Bao S et al (2020) Remarkable switching of transport properties and surface exchange kinetics in epitaxial PrBaMn2O5+δ films. Acta Mater 186:517–522. https://doi.org/10.1016/j.actamat.2020.01.029
Pineda OL, Moreno ZL, Roussel P et al (2016) Synthesis and preliminary study of the double perovskite NdBaMn2O5+δ as symmetric SOFC electrode material. Solid State Ion 288:61–67. https://doi.org/10.1016/j.ssi.2016.01.022
Sun YF, Zhang YQ, Hua B et al (2016) Molybdenum doped Pr0.5Ba0.5MnO3−δ (Mo-PBMO) double perovskite as a potential solid oxide fuel cell anode material. J Power Sources 301:237–241. https://doi.org/10.1016/j.jpowsour.2015.09.127
Felli A, Mauri S, Marelli M et al (2022) Insights into the redox behavior of Pr0.5Ba0.5MnO3-d - derived perovskites for CO2 valorization technologies. ACS Appl Energy Mater. https://doi.org/10.1021/acsaem.2c00163
Hou N, Li P, Lv T et al (2017) Sm0.5Ba0.5MnO3−δ anode for solid oxide fuel cells with hydrogen and methanol as fuels. Catal Today 298:33–39. https://doi.org/10.1016/j.cattod.2017.06.034
Tsuji E, Motohashi T, Noda H et al (2018) Strong lanthanoid substitution effect on electrocatalytic activity of double-perovskite-type BaLnMn2O5 (Ln = Y, Gd, Nd, and La) for oxygen reduction reaction. J Phys Chem C 122:7081–7087. https://doi.org/10.1021/acs.jpcc.7b12678
Wang J, Gao Y, Chen D et al (2018) Water splitting with an enhanced bifunctional double perovskite. ACS Catal 8:364–371. https://doi.org/10.1021/acscatal.7b02650
Alonso JA, Martínez-Lope MJ, Casais MT, Fernández-Díaz MT (2000) Evolution of the Jahn-Teller distortion of MnO6 octahedra in RMnO3 perovskites (R = Pr, Nd, Dy, Tb, Ho, Er, Y): a neutron diffraction study. Inorg Chem 39:917–923. https://doi.org/10.1021/ic990921e
Trukhanov SV, Lobanovski LS, Bushinsky MV et al (2003) Magnetic phase transitions in the anion-deficient. J Phys Condens Matter 15:1783–1795. https://doi.org/10.1088/0953-8984/15/10/324
Trukhanov SV, Trukhanov AV, Szymczak H et al (2006) Thermal stability of A-site ordered PrBaMn2O6 manganites. J Phys Chem Solids 67:675–681. https://doi.org/10.1016/j.jpcs.2005.09.099
King G, Woodward PM (2010) Cation ordering in perovskites. J Mater Chem 20:5785–5796. https://doi.org/10.1039/b926757c
Raveau B, Caignaert V, Kundu AK (2015) Double cationic-anionic ordering in Ba-based oxygen-deficient perovskites. Z Anorg Allg Chem 641:990–997. https://doi.org/10.1002/zaac.201500088
Talanov VM, Talanov MV, Shirokov VB (2014) Group-theoretical study of cationic ordering in perovskite structure. Crystallogr Rep 59:650–661. https://doi.org/10.1134/S1063774514050186
Taskin AA, Lavrov AN, Ando Y (2007) Fast oxygen diffusion in A-site ordered perovskites. Prog Solid State Chem 35:481–490. https://doi.org/10.1016/j.progsolidstchem.2007.01.014
Urusova AS, Cherepanov VA, Lebedev OI et al (2014) Tuning oxygen content and distribution by substitution at Co site in 112 YBaCo2O5+δ: impact on transport and thermal expansion properties. J Mater Chem A 2:8823–8832. https://doi.org/10.1039/c4ta01264j
Leonidov IA, Markov AA, Zavyalov MA et al (2022) Structural features and defect equilibrium in cubic PrBa1−xSrxFe2O6−δ. Materials 15:4390. https://doi.org/10.3390/ma15134390
Gilleßen M, Marck L, George J et al (2012) Oxygen-storage materials BaYMn2O5+δ from the quantum-chemical. Chem Mater 24:1910–1916. https://doi.org/10.1021/cm300655y
Shin TH, Myung JH, Verbraeken M et al (2015) Oxygen deficient layered double perovskite as an active cathode for CO2 electrolysis using a solid oxide conductor. Faraday Discuss 182:227–239. https://doi.org/10.1039/c5fd00025d
Motohashi T, Kimura M, Masubuchi Y et al (2016) Significant lanthanoid substitution effect on the redox reactivity of the oxygen-storage material BaYMn2O5+δ. Chem Mater 28:4409–4414. https://doi.org/10.1021/acs.chemmater.6b01501
Trukhanov SV, Troyanchuk IO, Trukhanov AV, Szymczak H (2007) Effect of the A-site randomness on magnetotransport properties of PrBaMn2O6 manganites. Solid State Phenom 128:187–192. https://doi.org/10.4028/www.scientific.net/SSP.128.187
Trukhanov SV, Lobanovski LS, Bushinsky MV et al (2005) Study of A-site ordered PrBaMn2O6-δ manganite properties depending on the treatment conditions. J Phys Condens Matter 17:6495–6506. https://doi.org/10.1088/0953-8984/17/41/019
Trukhanov SV, Troyanchuk IO, Hervieu M et al (2002) Magnetic and electrical properties of LBaMn2O6-γ (Nd, Sm, Eu, Gd, Tb) manganites. Phys Rev B Condens Matter Mater Phys 66:1–10. https://doi.org/10.1103/PhysRevB.66.184424
Gilev AR, Kiselev EA, Mychinko MY, Cherepanov VA (2019) Topotactic synthesis, crystal structure and oxygen non-stoichiometry of ordered NdBaMnFeO6−δ. Mater Res Bull 113:1–5. https://doi.org/10.1016/j.materresbull.2018.12.037
Mostovshchikova EV, Sterkhov EV, Naumov SV et al (2021) Effect of A-site ordering on IR absorption and magnetotransmission in PrBaMn2O6 double manganite. J Magn Magn Mater 538:168247. https://doi.org/10.1016/j.jmmm.2021.168247
Zhang Q, Guillou F, Wahl A et al (2010) Coexistence of inverse and normal magnetocaloric effect in A-site ordered NdBaMn2O6. Appl Phys Lett 96:7–10. https://doi.org/10.1063/1.3453657
Yamada S, Sagayama H, Higuchi K et al (2017) Physical properties and crystal structure analysis of double-perovskite NdBaMn2O6 by using single crystals. Phys Rev B 95:1–7. https://doi.org/10.1103/PhysRevB.95.035101
Troyanchuk IO, Trukhanov SV, Szymczak G (2002) New family of LnBaMn2O6−γ manganites (Ln = Nd, Sm, and Gd). Crystallogr Rep 47:658–665. https://doi.org/10.1134/1.1496067
Ling DC, Hsu PC, Lee CL (2012) Correlation between A-site randomness and magnetic phase transition in half-doped manganite Pr0.5Ba0.5MnO3. J Phys Conf Ser 400:9–13. https://doi.org/10.1088/1742-6596/400/3/032047
Nakajima T, Yoshizawa H, Ueda Y (2004) A-site randomness effect on structural and physical properties of Ba-based perovskite manganites. J Phys Soc Jpn 73:2283–2291. https://doi.org/10.1143/JPSJ.73.2283
Martinez-Rodriguez HA, Jurado JF, Herrera-Pérez G et al (2021) Enhancing Pr1−xBaxMnO3−δ perovskite charge-transport by electronic structure modulation. J Mater Sci 56:16510–16523. https://doi.org/10.1007/s10853-021-06332-z
Trukhanov SV, Khomchenko VA, Karpinsky DV et al (2019) A-site ordered state in manganites with perovskite-like structure based on optimally doped compounds Ln0.70Ba0.30MnO3 (Ln = Pr, Nd). J Rare Earths 37:1242–1249. https://doi.org/10.1016/j.jre.2018.12.010
Trukhanov SV (2005) Investigation of stability of ordered manganites. J Exp Theor Phys 101:513–520. https://doi.org/10.1134/1.2103220
Nakajima T, Kageyama H, Yoshizawa H, Ueda Y (2002) Structures and electromagnetic properties of new metal-ordered manganites: RBaMn2O6 (R = Y and rare-earth elements). J Phys Soc Jpn 71:2843–2846. https://doi.org/10.1143/JPSJ.71.2843
Nakajiama T, Kageyama H, Ueda Y (2003) Structures and physical properties of metal-ordered manganites RBaMn2O6 (R: Y and rare earth elements). Phys B 329–333:844–845. https://doi.org/10.1016/S0921-4526(02)02545-0
Mero RD, Ogawa K, Yamada S, Liu HL (2019) Optical study of the electronic structure and lattice dynamics of NdBaMn2O6 single crystals. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-54524-0
Yamada S, Sagayama H, Sugimoto K, Arima T (2018) Successive phase transitions and magnetic fluctuation in a double-perovskite NdBaMn2O6 single crystal. J Phys Conf Ser. https://doi.org/10.1088/1742-6596/969/1/012103
Sterkhov EV, Uporov SA (2018) Transformation of magnetic transitions and crystal structure of NdBaMn2O6 at diamagnetic dilution with Ti4+ ions in the Mn-sublattice. J Struct Chem 59:2023–2028. https://doi.org/10.1134/S002247661808036X
Yamada S, Abe N, Sagayama H et al (2019) Room-temperature low-field colossal magnetoresistance in double-perovskite manganite. Phys Rev Lett 123:1–6. https://doi.org/10.1103/PhysRevLett.123.126602
Zhang Y, Zhao H, Du Z et al (2019) High-performance SmBaMn2O5+δ electrode for symmetrical solid oxide fuel cell. Chem Mater 31:3784–3793. https://doi.org/10.1021/acs.chemmater.9b01012
Xu X, Zhong Y, Shao Z (2019) Double perovskites in catalysis, electrocatalysis, and photo(electro)catalysis. Trends Chem 1:410–424. https://doi.org/10.1016/j.trechm.2019.05.006
Chen D, Wang J, Zhang Z et al (2016) Boosting oxygen reduction/evolution reaction activities with layered perovskite catalysts. Chem Commun 52:10739–10742. https://doi.org/10.1039/c6cc04895a
Rodriguez-Carvajal J (1993) Recent advances in magnetic structure determination by neutron powder diffraction. Phys B 192:55–69. https://doi.org/10.1016/0921-4526(93)90108-I
Shalamova AM, Glazyrina YA, Suntsov AY (2022) Elevated electrochemical activity of double perovskites PrBaCo2−xNixO6−δ towards hydrogen peroxide oxidation. J Electroanal Chem 905:115959. https://doi.org/10.1016/j.jelechem.2021.115959
Tonus F, Bahout M, Dorcet V et al (2017) A-site order-disorder in the NdBaMn2O5+δ SOFC electrode material monitored in-situ by neutron diffraction under hydrogen flow. J Mater Chem A 5:11078–11085. https://doi.org/10.1039/c7ta01439b
Shalamova AM, Suntsov AY (2022) Synthesis features and determination of oxygen content in double perovskite-like manganites LnBaMn2O6−δ (Ln = Nd, Sm). In: AIP Conference Proceedings, vol 2466
Klimkowicz A, Świerczek K, Rząsa T et al (2016) Oxygen storage properties and catalytic activity of layer-orderedperovskites BaY1−xGdxMn2O5+δ. Solid State Ion 288:43–47. https://doi.org/10.1016/j.ssi.2016.01.038
Felli A, Trovarelli A, Boaro M (2021) Investigation of the redox behaviour of double perovskite PrBaMn2O5+δ. ECS Meet Abstr. https://doi.org/10.1149/ma2021-031134mtgabs
Tomkiewicz AC, Tamimi MA, Huq A, McIntosh S (2016) Structural analysis of PrBaMn2O5+δ under SOFC anode conditions by in-situ neutron powder diffraction. J Power Sources 330:240–245. https://doi.org/10.1016/j.jpowsour.2016.09.013
Kwon O, Sengodan S, Kim K et al (2017) Exsolution trends and co-segregation aspects of self-grown catalyst nanoparticles in perovskites. Nat Commun 8:1–7. https://doi.org/10.1038/ncomms15967
Wang WR, Xu DP, Su WH (2005) Raman shift of RMnO3 (R = La, Pr, Nd, Sm) manganites. Chin Phys Lett 22:705–707. https://doi.org/10.1088/0256-307X/22/3/051
Nakajima T, Kageyama H, Yoshizawa H et al (2003) Ground state properties of the A-site ordered manganites, RBaMn2O6 (R = La, Pr and Nd). J Phys Soc Jpn 72:3237–3242. https://doi.org/10.1143/JPSJ.72.3237
Sengodan S, Choi S, Jun A et al (2015) Layered oxygen-deficient double perovskite as an efficient and stable anode for direct hydrocarbon solid oxide fuel cells. Nat Mater 14:205–209. https://doi.org/10.1038/nmat4166
Klimkowicz A, Świerczek K, Takasaki A et al (2015) Crystal structure and oxygen storage properties of BaLnMn2O5+δ (Ln: Pr, Nd, Sm, Gd, Dy, Er and Y) oxides. Mater Res Bull 65:116–122. https://doi.org/10.1016/j.materresbull.2015.01.041
Klimkowicz A, Świerczek K, Zheng K et al (2017) Oxygen release from BaLnMn2O6 (Ln: Pr, Nd, Y) under reducing conditions as studied by neutron diffraction. J Mater Sci 52:6476–6485. https://doi.org/10.1007/s10853-017-0883-2
Autret C, Maignan A, Martin C et al (2003) Magnetization steps in a noncharge-ordered manganite, Pr0.5Ba0.5MnO3. Appl Phys Lett 82:4746–4746. https://doi.org/10.1063/1.1588756
Świerczek K, Klimkowicz A, Zheng K, Dabrowski B (2013) Synthesis, crystal structure and electrical properties of A-site cation ordered BaErMn2O5 and BaErMn2O6. J Solid State Chem 203:68–73. https://doi.org/10.1016/j.jssc.2013.04.010
Klimkowicz A, Świerczek K, Yamazaki T, Takasaki A (2016) Enhancement of the oxygen storage properties of BaPrMn2O5+δ and BaSmMn2O5+δ oxides by a high-energy milling. Solid State Ionics 298:66–72. https://doi.org/10.1016/j.ssi.2016.11.013
Motohashi T, Ueda T, Masubuchi Y, Kikkawa S (2011) Enhanced oxygen intake/release kinetics of BaYMn2O5+δ fine powders prepared by a wet-chemical route. J Ceram Soc Jpn 119:894–897. https://doi.org/10.2109/jcersj2.119.894
Acknowledgements
This work is partly supported by the Russian Science Foundation under grant of № 22-19-00129. All persons who took any part in the preparation of this article and the performance of experiments are listed as co-authors.
Author information
Authors and Affiliations
Contributions
A.M.S: Methodology, Investigation, Data curation, Conceptualization, Formal analysis, Visualization, Writing—original draft. R.F.S: Methodology, Investigation. A.V.C: Methodology, Investigation. A.Y.S: Conceptualization, Formal analysis, Funding acquisition, Writing—review & editing, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not Applicable.
Additional information
Handling Editor: David Cann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shalamova, A.M., Samigullina, R.F., Chukin, A.V. et al. Evolution of crystal structure and redox activity of LnBaMn2O6−δ upon various external conditions: in-situ characterization. J Mater Sci 58, 16634–16650 (2023). https://doi.org/10.1007/s10853-023-09060-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-09060-8