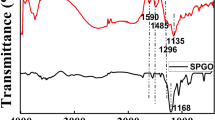
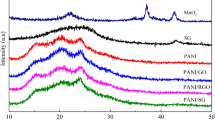
Abstract
A series of graphene materials are prepared by intercalation of graphene oxide (GO) with different surfactants, cetyltrimethylammonium bromide (CTAB), n-octyltrimethylammonium bromide, tetramethylammonium bromide, and sodium dodecylbenzene sulfonate, subsequently by γ-ray induced reduction in N-methyl-2-pyrrolidone (NMP) at room temperature. GO can be reduced by the electrons generated from the radiolysis of NMP under γ-ray irradiation, and reduced GO is simultaneously functionalized by the radiolytic product of NMP. Cationic surfactant CTAB with longer alkyl chains can effectively promote the reduction process of GO by preventing the aggregation of graphene sheets, which has been testified by X-ray photoelectron spectroscopy, X-ray diffraction, thermogravimetric analysis, Raman spectroscopy, and Fourier transform infrared spectroscopy analyses. Furthermore, when the as-prepared graphene/polyaniline composites are used for supercapacitor electrode materials, there is a highest specific capacitance of 484 F g−1 at a current density of 0.1 A g−1 for the graphene produced in the presence of cationic surfactant CTAB.






Similar content being viewed by others
References
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
Novoselov KS, Fal’ko VI, Colombo L, Gellert PR, Schwab MG, Kim K (2012) A roadmap for graphene. Nature 490:192–200
Pumera M (2011) Graphene-based nanomaterials for energy storage. Energy Environ Sci 4:668–674
Huang X, Yin ZY, Wu SX, Qi XY, He QY, Zhang QC, Yan QY, Boey F, Zhang H (2011) Graphene-based materials: synthesis, characterization, properties, and applications. Small 7:1876–1902
de Guzman RC, Yang JH, Ming-Cheng M, Salley SO, Ng KYS (2013) A silicon nanoparticle/reduced graphene oxide composite anode with excellent nanoparticle dispersion to improve lithium ion battery performance. J Mater Sci 48:4823–4833. doi:10.1007/s10853-012-7094-7
Liu GJ, Fan LQ, Yu FD, Wu JH, Liu L, Qiu ZY, Liu Q (2013) Facile one-step hydrothermal synthesis of reduced graphene oxide/Co3O4 composites for supercapacitors. J Mater Sci 48:8463–8470. doi:10.1007/s10853-013-7663-4
Zhang Y, Zhang LY, Zhou CW (2013) Review of chemical vapor deposition of graphene and related applications. Acc Chem Res 46:2329–2339
Berger C, Song ZM, Li XB, Wu XS, Brown N, Naud C, Mayou D, Li TB, Hass J, Marchenkov AN, Conrad EH, First PN, de Heer WA (2006) Electronic confinement and coherence in patterned epitaxial graphene. Science 312:1191–1196
Guardia L, Fernandez-Merino MJ, Paredes JI, Solis-Fernandez P, Villar-Rodil S, Martinez-Alonso A, Tascon JMD (2011) High-throughput production of pristine graphene in an aqueous dispersion assisted by non-ionic surfactants. Carbon 49:1653–1662
Pei SF, Cheng HM (2012) The reduction of graphene oxide. Carbon 50:3210–3228
Stankovich S, Piner RD, Chen XQ, Wu NQ, Nguyen ST, Ruoff RS (2006) Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate). J Mater Chem 16:155–158
Xu YX, Bai H, Lu GW, Li C, Shi GQ (2008) Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J Am Chem Soc 130:5856–5857
Stankovich S, Piner RD, Nguyen ST, Ruoff RS (2006) Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 44:3342–3347
Zhang K, Mao L, Zhang LL, Chan HSO, Zhao XS, Wu JS (2011) Surfactant-intercalated, chemically reduced graphene oxide for high performance supercapacitor electrodes. J Mater Chem 21:7302–7307
Chang HX, Wang GF, Yang A, Tao XM, Liu XQ, Shen YD, Zheng ZJ (2010) A transparent, flexible, low-temperature, and solution-processible graphene composite electrode. Adv Funct Mater 20:2893–2902
Zhang JL, Yang HJ, Shen GX, Cheng P, Zhang JY, Guo SW (2010) Reduction of graphene oxide via L-ascorbic acid. Chem Commun 46:1112–1114
Chen DZ, Li LD, Guo L (2011) An environment-friendly preparation of reduced graphene oxide nanosheets via amino acid. Nanotechnology 22:325601–325607
Bose S, Kuila T, Mishra AK, Kim NH, Lee JH (2012) Dual role of glycine as a chemical functionalizer and a reducing agent in the preparation of graphene: an environmentally friendly method. J Mater Chem 22:9696–9703
Zhang YW, Ma HL, Zhang QL, Peng J, Li JQ, Zhai ML, Yu ZZ (2012) Facile synthesis of well-dispersed graphene by gamma-ray induced reduction of graphene oxide. J Mater Chem 22:13064–13069
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Ma HL, Zhang YW, Hu QH, Yan D, Yu ZZ, Zhai ML (2012) Chemical reduction and removal of Cr(VI) from acidic aqueous solution by ethylenediamine-reduced graphene oxide. J Mater Chem 22:5914–5916
Kim J, Cote LJ, Kim F, Yuan W, Shull KR, Huang JX (2010) Graphene oxide sheets at interfaces. J Am Chem Soc 132:8180–8186
Liang YY, Wu DQ, Feng XL, Mullen K (2009) Dispersion of graphene sheets in organic solvent supported by ionic interactions. Adv Mater 21:1679–1683
Shen JF, Hu YZ, Shi M, Lu X, Qin C, Li C, Ye MX (2009) Fast and facile preparation of graphene oxide and reduced graphene oxide nanoplatelets. Chem Mater 21:3514–3520
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Wu Y, Nguyen ST, Ruoff RS (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45:1558–1565
Tuinstra F, Koenig JL (1970) Raman spectrum of graphite. J Chem Phys 53:1126–1130
Moon GH, Park Y, Kim W, Choi W (2011) Photochemical loading of metal nanoparticles on reduced graphene oxide sheets using phosphotungstate. Carbon 49:3454–3462
Zhang QL, Zhang YW, Gao ZH, Ma HL, Wang SJ, Peng J, Li JQ, Zhai ML (2013) A facile synthesis of platinum nanoparticle decorated graphene by one-step gamma-ray induced reduction for high rate supercapacitors. J Mater Chem C 1:321–328
Pham VH, Cuong TV, Hur SH, Oh E, Kim EJ, Shin EW, Chung JS (2011) Chemical functionalization of graphene sheets by solvothermal reduction of a graphene oxide suspension in N-methyl-2-pyrrolidone. J Mater Chem 21:3371–3377
Wang SJ, Zhang YW, Ma HL, Zhang QL, Xu WG, Peng J, Li JQ, Yu ZZ, Zhai ML (2013) Ionic-liquid-assisted facile synthesis of silver nanoparticle-reduced graphene oxide hybrids by gamma irradiation. Carbon 55:245–252
Mayavan S, Sim JB, Choi SM (2012) Easy synthesis of nitrogen-doped graphene-silver nanoparticle hybrids by thermal treatment of graphite oxide with glycine and silver nitrate. Carbon 50:5148–5155
Cui P, Lee J, Hwang E, Lee H (2011) One-pot reduction of graphene oxide at subzero temperatures. Chem Commun 47:12370–12372
Ferrari AC, Basko DM (2013) Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat Nanotechnol 8:235–246
Lin ZY, Yao YG, Li Z, Liu Y, Li Z, Wong CP (2010) Solvent-assisted thermal reduction of graphite oxide. J Phys Chem C 114:14819–14825
Park S, An JH, Jung IW, Piner RD, An SJ, Li XS, Velamakanni A, Ruoff RS (2009) Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents. Nano Lett 9:1593–1597
Yang DX, Velamakanni A, Bozoklu G, Park S, Stoller M, Piner RD, Stankovich S, Jung I, Field DA, Ventrice CA, Ruoff RS (2009) Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 47:145–152
Qiu Y, Gao L (2005) Preparation and characterization of CrN/CN and nano-TiN/CN composites. J Am Ceram Soc 88:494–496
Tan S, Belanger D (2005) Characterization and transport properties of Nafion/polyaniline composite membranes. J Phys Chem B 109:23480–23490
Zhou XS, Wu TB, Hu BJ, Yang GY, Han BX (2010) Synthesis of graphene/polyaniline composite nanosheets mediated by polymerized ionic liquid. Chem Commun 46:3663–3665
Dubin S, Gilje S, Wang K, Tung VC, Cha K, Hall AS, Farrar J, Varshneya R, Yang Y, Kaner RB (2010) A one-step, solvothermal reduction method for producing reduced graphene oxide dispersions in organic solvents. ACS Nano 4:3845–3852
Liu KJ, Salmon GA (1992) Radiation chemistry of organic amides. Part 3. Yield and mechanism of formation of molecular hydrogen in N-methylpyrrolidin-2-one. J Chem Soc Faraday Trans 88:1255–1259
Tung TT, Kim TY, Shim JP, Yang WS, Kim H, Suh KS (2011) Poly(ionic liquid)-stabilized graphene sheets and their hybrid with poly(3,4-ethylenedioxythiophene). Org Electron 12:2215–2224
Tang HX, Ehlert GJ, Lin YR, Sodano HA (2012) Highly efficient synthesis of graphene nanocomposites. Nano Lett 12:84–90
Acik M, Lee G, Mattevi C, Chhowalla M, Cho K, Chabal YJ (2010) Unusual infrared-absorption mechanism in thermally reduced graphene oxide. Nat Mater 9:840–845
Yan J, Wei T, Shao B, Fan ZJ, Qian WZ, Zhang ML, Wei F (2010) Preparation of a graphene nanosheet/polyaniline composite with high specific capacitance. Carbon 48:487–493
Xu YF, Schwab MG, Strudwick AJ, Hennig I, Feng XL, Wu ZS, Mullen K (2013) Screen-printable thin film supercapacitor device utilizing graphene/polyaniline inks. Adv Energy Mater 3:1035–1040
Fan W, Zhang C, Tjiu WW, Pramoda KP, He CB, Liu TX (2013) Graphene-wrapped polyaniline hollow spheres as novel hybrid electrode materials for supercapacitor applications. ACS Appl Mater Inter 5:3382–3391
Wu Q, Xu YX, Yao ZY, Liu AR, Shi GQ (2010) Supercapacitors based on flexible graphene/polyaniline nanofiber composite films. ACS Nano 4:1963–1970
Wang DW, Li F, Zhao JP, Ren WC, Chen ZG, Tan J, Wu ZS, Gentle I, Lu GQ, Cheng HM (2009) Fabrication of graphene/polyaniline composite paper via in situ anodic electropolymerization for high-performance flexible electrode. ACS Nano 3:1745–1752
Kumar NA, Choi HJ, Shin YR, Chang DW, Dai LM, Baek JB (2012) Polyaniline-grafted reduced graphene oxide for efficient electrochemical supercapacitors. ACS Nano 6:1715–1723
Acknowledgements
The National Natural Science Foundation of China (NNSFC, Project No. 11375019) and the Bei**g Natural Science Foundation (Project No. 2132031) are acknowledged for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10853_2014_8286_MOESM1_ESM.doc
The online version of this article contains supplementary material, which is available to authorized users. (DOC 2365 kb)
Rights and permissions
About this article
Cite this article
Cai, X., Zhang, Q., Wang, S. et al. Surfactant-assisted synthesis of reduced graphene oxide/polyaniline composites by gamma irradiation for supercapacitors. J Mater Sci 49, 5667–5675 (2014). https://doi.org/10.1007/s10853-014-8286-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8286-0