Abstract
Background
Granulosa cell (GC) proliferation and apoptosis are critical events of the ovum energy supply, which lead to follicular growth retardation or atresia, and various ovulatory obstacles, eventually resulting in the development of ovarian disorders such as polycystic ovarian syndrome (PCOS). Apoptosis and dysregulated miRNA expression in GCs are manifestations of PCOS. miR-4433a-3p has been reported to be involved in apoptosis. However, there is no study reporting the roles of miR-4433a-3p in GC apoptosis and PCOS progression.
Methods
miR-4433a-3p and peroxisome proliferator–activated receptor alpha (PPAR-α) levels in GCs of PCOS patients or in tissues of a PCOS rat model were examined by quantitative polymerase chain reaction and immunohistochemistry. Bioinformatics analyses and luciferase assays were used to examine the association between miR-4433a-3p and PPAR-α, as well as PPAR-α and immune cell infiltration, in PCOS patients.
Results
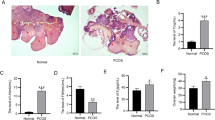
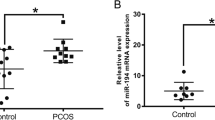
miR-4433a-3p expression in GCs of PCOS patients was increased. miR-4433a-3p overexpression inhibited the growth of the human granulosa-like tumor cell line (KGN) and promoted apoptosis, while co-treatment with PPAR-α and miR-4433a-3p mimic rescued miR-4433a-3p-induced apoptosis. PPAR-α was a direct target of miR-4433a-3p and its expression was decreased in PCOS patients. PPAR-α expression was also positively correlated with the infiltration of activated CD4+ T cells, eosinophils, B cells, gamma delta T cells, macrophages, and mast cells, but negatively correlated with the infiltration of activated CD8+ T cells, CD56+ bright natural killer cells, immature dendritic cells, monocytes, plasmacytoid dendritic cells, neutrophils, and type 1 T helper cells in PCOS patients.
Conclusion
The miR-4433a-3p/PPAR-α/immune cell infiltration axis may function as a novel cascade to alter GC apoptosis in PCOS.






Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request, and in the Gene Expression Omnibus (GEO) repository. GSE84376 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE84376).
References
Nandi A, Chen Z, Patel R, Poretsky L. Polycystic ovary syndrome. Endocrinol Metab Clin North Am. 2014;43(1):123–47.
Li Y, Zheng Q, Sun D, et al. Dehydroepiandrosterone stimulates inflammation and impairs ovarian functions of polycystic ovary syndrome. J Cell Physiol. 2019;234(5):7435–47.
Rostamtabar M, Esmaeilzadeh S, Tourani M, et al. Pathophysiological roles of chronic low-grade inflammation mediators in polycystic ovary syndrome. J Cell Physiol. 2021;236(2):824–38.
Jeanes YM, Reeves S. Metabolic consequences of obesity and insulin resistance in polycystic ovary syndrome: diagnostic and methodological challenges. Nutr Res Rev. 2017;30(1):97–105.
Zheng Q, Li Y, Zhang D, et al. ANP promotes proliferation and inhibits apoptosis of ovarian granulosa cells by NPRA/PGRMC1/EGFR complex and improves ovary functions of PCOS rats. Cell Death Dis. 2017;8(10):e3145.
Lin M, Hua R, Ma J, et al. Bisphenol A promotes autophagy in ovarian granulosa cells by inducing AMPK/mTOR/ULK1 signalling pathway. Environ Int. 2021;147:106298.
Imbar T, Eisenberg I. Regulatory role of microRNAs in ovarian function. Fertil Steril. 2014;101(6):1524–30.
Stahlhut C, Slack FJ. MicroRNAs and the cancer phenotype: profiling, signatures and clinical implications. Genome Med. 2013;5(12):111.
Fu X, He Y, Wang X, et al. MicroRNA-16 promotes ovarian granulosa cell proliferation and suppresses apoptosis through targeting PDCD4 in polycystic ovarian syndrome. Cell Physiol Biochem. 2018;48(2):670–82.
Robinson CL, Zhang L, Schutz LF, Totty ML, Spicer LJ. MicroRNA 221 expression in theca and granulosa cells: hormonal regulation and function. J Anim Sci. 2018;96(2):641–52.
Kong F, Du C, Wang Y. MicroRNA-9 affects isolated ovarian granulosa cells proliferation and apoptosis via targeting vitamin D receptor. Mol Cell Endocrinol. 2019;486:18–24.
Tesfaye D, Gebremedhn S, Salilew-Wondim D, et al. MicroRNAs: tiny molecules with a significant role in mammalian follicular and oocyte development. Reproduction. 2018;155(3):R121–35.
Peng L, Chen Z, Chen Y, Wang X, Tang N. MIR155HG is a prognostic biomarker and associated with immune infiltration and immune checkpoint molecules expression in multiple cancers. Cancer Med. 2019;8(17):7161–73.
Yuan X, Berg N, Lee JW, et al. MicroRNA miR-223 as regulator of innate immunity. J Leukoc Biol. 2018;104(3):515–24.
Bougarne N, Weyers B, Desmet SJ, et al. Molecular actions of PPARalpha in lipid metabolism and inflammation. Endocr Rev. 2018;39(5):760–802.
Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62(3):720–33.
Preidis GA, Kim KH, Moore DD. Nutrient-sensing nuclear receptors PPARalpha and FXR control liver energy balance. J Clin Invest. 2017;127(4):1193–201.
San-Millan JL, Escobar-Morreale HF. The role of genetic variation in peroxisome proliferator-activated receptors in the polycystic ovary syndrome (PCOS): an original case-control study followed by systematic review and meta-analysis of existing evidence. Clin Endocrinol (Oxf). 2010;72(3):383–92.
Yazawa T, Inaoka Y, Okada R, et al. PPAR-gamma coactivator-1alpha regulates progesterone production in ovarian granulosa cells with SF-1 and LRH-1. Mol Endocrinol. 2010;24(3):485–96.
Zhu Q, Zuo R, He Y, et al. Local regeneration of cortisol by 11beta-HSD1 contributes to insulin resistance of the granulosa cells in PCOS. J Clin Endocrinol Metab. 2016;101(5):2168–77.
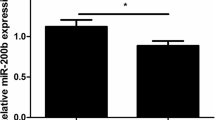
He T, Sun Y, Zhang Y, et al. MicroRNA-200b and microRNA-200c are up-regulated in PCOS granulosa cell and inhibit KGN cell proliferation via targeting PTEN. Reprod Biol Endocrinol. 2019;17(1):68.
Liu H, **e J, Fan L, et al. Cryptotanshinone protects against PCOS-induced damage of ovarian tissue via regulating oxidative stress, mitochondrial membrane potential, inflammation, and apoptosis via regulating ferroptosis. Oxid Med Cell Longev. 2022;2022:8011850.
Song J, Luo S, Li SW. miRNA-592 is downregulated and may target LHCGR in polycystic ovary syndrome patients. Reprod Biol. 2015;15(4):229–37.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8.
Dorostghoal M, Mahabadi MK, Adham S. Effects of maternal caffeine consumption on ovarian follicle development in wistar rats offspring. J Reprod Infertil. 2011;12(1):15–22.
Geng X, Zhao J, Huang J, et al. lnc-MAP3K13-7:1 inhibits ovarian GC proliferation in PCOS via DNMT1 downregulation-mediated CDKN1A promoter hypomethylation. Mol Ther. 2021;29(3):1279–93.
Liu Y, Liu H, Li Z, et al. The release of peripheral immune inflammatory cytokines promote an inflammatory cascade in PCOS patients via altering the follicular microenvironment. Front Immunol. 2021;12:685724.
Dai D, Tan Y, Guo L, Tang A, Zhao Y. Identification of exosomal miRNA biomarkers for diagnosis of papillary thyroid cancer by small RNA sequencing. Eur J Endocrinol. 2020;182(1):111–21.
Wu H, Yin J, Ai Z, Li G, Li Y, Chen L. Overexpression of miR-4433 by suberoylanilide hydroxamic acid suppresses growth of CML cells and induces apoptosis through targeting Bcr-Abl. J Cancer. 2019;10(23):5671–80.
Gong Y, Luo S, Fan P, Zhu H, Li Y, Huang W. Growth hormone activates PI3K/Akt signaling and inhibits ROS accumulation and apoptosis in granulosa cells of patients with polycystic ovary syndrome. Reprod Biol Endocrinol. 2020;18(1):121.
Liu Y, Zhai J, Chen J, Wang X, Wen T. PGC-1alpha protects against oxidized low-density lipoprotein and luteinizing hormone-induced granulosa cells injury through ROS-p38 pathway. Hum Cell. 2019;32(3):285–96.
Xu P, Gildea JJ, Zhang C, et al. Stomach gastrin is regulated by sodium via PPAR-alpha and dopamine D1 receptor. J Mol Endocrinol. 2020;64(2):53–65.
Komar CM. Peroxisome proliferator-activated receptors (PPARs) and ovarian function–implications for regulating steroidogenesis, differentiation, and tissue remodeling. Reprod Biol Endocrinol. 2005;3:41.
Sato N, Uchida K, Nakajima M, Watanabe A, Kohira T. Collaborative work on evaluation of ovarian toxicity. 13) Two- or four-week repeated dose studies and fertility study of PPAR alpha/gamma dual agonist in female rats. J Toxicol Sci. 2009;34(Suppl 1):137–46.
Wang DR, Wang B, Yang M, et al. Suppression of miR-30a-3p attenuates hepatic steatosis in non-alcoholic fatty liver disease. Biochem Genet. 2020;58(5):691–704.
Brennan KM, Kroener LL, Chazenbalk GD, Dumesic DA. Polycystic ovary syndrome: impact of lipotoxicity on metabolic and reproductive health. Obstet Gynecol Surv. 2019;74(4):223–31.
Lovekamp-Swan T, Jetten AM, Davis BJ. Dual activation of PPARalpha and PPARgamma by mono-(2-ethylhexyl) phthalate in rat ovarian granulosa cells. Mol Cell Endocrinol. 2003;201(1–2):133–41.
Froment P, Gizard F, Defever D, Staels B, Dupont J, Monget P. Peroxisome proliferator-activated receptors in reproductive tissues: from gametogenesis to parturition. J Endocrinol. 2006;189(2):199–209.
Fuhler GM. The immune system and microbiome in pregnancy. Best Pract Res Clin Gastroenterol. 2020;44–45:101671.
Zhang X, Zhivaki D, Lo-Man R. Unique aspects of the perinatal immune system. Nat Rev Immunol. 2017;17(8):495–507.
Pires MA, Payan-Carreira R. Resident macrophages and lymphocytes in the canine endometrium. Reprod Domest Anim. 2015;50(5):740–9.
Cornish EF, Filipovic I, Asenius F, Williams DJ, McDonnell T. Innate immune responses to acute viral infection during pregnancy. Front Immunol. 2020;11:572567.
Gordon SM, Nishiguchi MA, Chase JM, Mani S, Mainigi MA, Behrens EM. IFNs drive development of novel IL-15-responsive macrophages. J Immunol. 2020;205(4):1113–24.
Zheng S, et al. Mechanism of quercetin on the improvement of ovulation disorder and regulation of ovarian CNP/NPR2 in PCOS model rats. J Formos Med Assoc. 2022;121(6):1081–92.
Krysiak R, Kowalcze K, Okopien B. Hypothalamic-pituitary-gonadal axis and sexual functioning in metformin-treated men after discontinuation of testosterone replacement therapy: a pilot study. J Clin Pharm Ther. 2021;46(6):1764–75.
Peng T, Phasouk K, Bossard E, et al. Distinct populations of antigen-specific tissue-resident CD8+ T cells in human cervix mucosa. JCI Insight. 2021; 6(15).
Kang S, Wu Q, Huang J, et al. Tissue resident memory gammadeltaT cells in murine uterus expressed high levels of IL-17 promoting the invasion of trophocytes. Front Immunol. 2020;11:588227.
Weigel K, Lobsien E, Lobsien D, Brodhun M, Conrad E, Steinbrecher A. Double vision and a wrong track. Fortschr Neurol Psychiatr. 2020;88(5):331–6.
Makker PG, Duffy SS, Lees JG, et al. Characterisation of immune and neuroinflammatory changes associated with chemotherapy-induced peripheral neuropathy. PLoS One. 2017;12(1):e0170814.
O’Dwyer KM, Advani AS. When to treat adults like children: optimizing therapy for lymphoblastic lymphoma in young adults. J Clin Oncol. 2016;34(6):533–8.
Matsuzaki J, Tsuji T, Chodon T, Ryan C, Koya RC, Odunsi K. A rare population of tumor antigen-specific CD4(+)CD8(+) double-positive alphabeta T lymphocytes uniquely provide CD8-independent TCR genes for engineering therapeutic T cells. J Immunother Cancer. 2019;7(1):7.
Huang X, Liu L, Xu C, et al. Tissue-resident CD8(+) T memory cells with unique properties are present in human decidua during early pregnancy. Am J Reprod Immunol. 2020;84(1):e13254.
Li Z, Peng A, Feng Y, et al. Detection of T lymphocyte subsets and related functional molecules in follicular fluid of patients with polycystic ovary syndrome. Sci Rep. 2019;9(1):6040.
Funding
This research was supported by the National Natural Science Foundation of China (No. 81801532).
Author information
Authors and Affiliations
Contributions
LZ and XY analyzed and interpreted the patient data regarding the PCOS. YM, MWC, and SCL performed the histological examination of the ovarian and animal experiment. HYL and JQL were major contributors in writing the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All study protocols were approved by the Institutional Ethics Committee of the Third Affiliated Hospital Guangzhou Medical University (Ethics Review Board, 2018NO:083; Guangzhou, China).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, L., Yao, X., Mo, Y. et al. miR-4433a-3p promotes granulosa cell apoptosis by targeting peroxisome proliferator–activated receptor alpha and inducing immune cell infiltration in polycystic ovarian syndrome. J Assist Reprod Genet 40, 1447–1459 (2023). https://doi.org/10.1007/s10815-023-02815-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02815-x