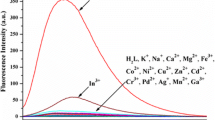
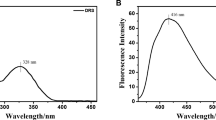
Aluminum is a widely distributed element and plays an indispensable role in our lives, but excessive intake of Al3+ can cause potential harm to human health. Highly selective methods for the rapid detection of Al3+ are urgently needed. Here, a novel "turn on" fluorescent probe L based on Schiff-base of 2-hydroxy-1-naphthaldehyde and 8-aminoquinoline was obtained in an excellent yield. Probe L presents significant properties such as rapid response, good stability, wide pH range, simple synthetic operation, and provides an efficient strategy for the highly selective detection of Al3+ in an aqueous solution of DMSO/H2O (7:3, v:v). According to Job's curve and fluorescence titration, probe L with Al3+ forms a 2:1 ratio of complex, and the lowest limit of detection is 3.23 × 10−8 mol/L. The possible combination mode for probe L with Al3+ was proposed by comparison with the changes of 1H NMR and FTIR spectra of L and its complex. In addition, the potent applicants for probe L were also investigated, and indicated that it could conveniently be made into a series of sensor strips and applied to rapidly detect trace Al3+ in traditional Chinese medicine.
Similar content being viewed by others
References
S. O. Tümay, Chem. Select., 6, 10561–10572 (2021).
S. Cichosz, A. Masek, and M. Zaborski, Polym. Test., 67, 342–348 (2018).
D. Sareen, P. Kaur, and K. Singh, Coord. Chem. Rev., 265, 125–154 (2014).
J. J. Torrez-Herrera, E. G. Fuentes-Ordoñez, and S. A. Korili, Powder Technol., 377, 80–88 (2021).
T. S. Liu, F. Qiu, B. X. Dong, R. Geng, M. Zha, H. Y. Yang, S. L. Shu, and Q. C. Jiang, Mater. Des., 206, Article ID 109743 (2021).
Y.-J. Liu, F.-F. Tian, X.-Y. Fan, F.-L. Jiang, and Y. I. Liu, Sens. Actuat. B: Chem., 240, 916–925 (2017).
K. P. Kepp, Chem. Rev., 112, 5193–5239 (2012).
S. V. Kumar, P. Kumara, and R. Gupta, New J. Chem., 44, 13285–13294 (2020).
H. N. Peng, Y. Q. Liu, J. Q. Huang, S. S. Huang, X. P. Cai, S. J. Xu, A. Q. Huang, and M. X. Zeng, J. Mol. Struct., 1229, Article ID 129866 (2021).
M. M. Wang, L. X. Lu, W. W. Song, X. Y. Wang, T. M. Sun, J. L. Zhu, J. Wang, J. Lumin., 233, Article ID 117911 (2021).
J. Hu, Y. Sun, J. Qi, P. Pei, Q. Lin, and Y.-M. Zhang, RSC Adv., 6, 100401–100406 (2016).
P. W. Cheah, M. P. Heng, A. Izati, C. H. Ng, and K. W. Tan, Inorg. Chim. Acta, 512, Article ID 119901 (2020).
S. Upadhyay, A. Singh, R. Sinha, S. Omer, and K. Negi, J. Mol. Struct., 1193, 89–102 (2019).
K. P. Carter, A. M. Young, and A. E. Palmer, Chem. Rev., 114, 4564–4601 (2014).
J. Berrones-Reyes, B. M. Muñoz-Flores, A. Gómez-Treviño, M. A. Treto-Suárez, D. Páez-Hernández, E. Schott, X. Zarate, and V. M. Jiménez-Pérez, Mater. Chem. Phys., 233, 89–101 (2019).
S. Mukherjee, S. Betal, and A. P. Chattopadhyay, New J. Chem., 44, 12692–12703 (2020).
S. Pramanik, S. K. Manna, S. Pathak, D. Mondal, K. Pal, and S. Mukhopadhyay, New J. Chem., 44, 13259–13265 (2020).
B. Balagurusamy, P. Ilayaperumal, Y. Zorlu, and R. Chellaiah, Chem. Select, 5, 8086–8092 (2020).
B. Bharali, H. Talukdar, P. Phukan, and D. K. Das, J. Fluoresc., 30, 751–757 (2020).
S. F. Carlos, R. F. Monteiro, D. L. A. Silva, C. Zanlorenzi, and F. S. Nunes, Spectrochim. Acta A: Mol. Biomol. Spectrosc., 231, Article ID 118119 (2020), https://doi.org/10.1016/j.saa.2020.118119.
S. Poomalai, S. Soundrapandian, S. Ramasamy, M. P. Selvakumar, and V. M. Enoch, IV, Chem. Phys. Lett., 751, Article ID 137551 (2020)
S. S. Peng, H. X. Wang, H. C. Ding, C. B. Fan, G. Liu, and S. Z. Pu, J. Photochem. Photobiol. A: Chem., 425, Article ID 113718 (2022).
Z. C. Zhou, W. J. Niu, Z. Q. Lin, Y. H. Cui, X. Tang, and Y. J. Li, Inorg. Chem. Commun., 121, Article ID 108168 (2020).
F.-F. Guo, B.-B. Wang, W.-N. Wu, W.-Y. Bi, Z.-H. Xu, Y.-C. Fan, L.-Y. Bian, and Y. Wang, J. Mol. Struct., 1251, Article ID 132073 (2022).
M. R. Wei, H. Q. Zhong, J. Zhou, W. D. Liu, W. B. **, P. F. Xu, Q. Q. Qiu, Z. S. Qian, and H. Feng, Talanta, 219, Article ID 121298 (2020).
L. Zhu, L. X. Lu, M. Wang, T. M. Sun, Y. Huang, C. X. Wang, W. Y. Bao, M. M. Wang, F. X. Zou, and Y. F. Tang, Tetrahedron Lett., 61, Article ID 151893 (2020).
G. Ganesan, B. Pownthurai, N. K. Kotwal, M. Yadav, P. Chetti, and A. Chaskar, J. Photochem. Photobiol. A: Chem., 425, 113699 (2022).
J. S. Heo, D. K. Gil,·and C. Kim, J. Fluoresc., 32, 825–833 (2022); https://doi.org/10.1007/s10895-021-02869-z.
H. J. Kim, B. Suh, and C. Kim, J. Chin. Chem. Soc., 69, 366–374 (2022).
S. Poomalai, S. Soundrapandian, S. Ramasamy, M. I. Selvakumar, V. Paulraj, and M. V. Enoch, Chem. Phys. Lett., 751, Article ID 137551 (2020).
L. Bai, Y. Xu, G. Li, S. Tian, L. Li, F. Tao, A. Deng, S. Wang, and L. Wang, Polymer, 11, 573 (2019).
J. M. Bak, S.-H. Jung, and H. I. Lee, Sens. Actuat. B Chem., 345, Article ID 130420 (2021).
K. Wu, J. Hu, X. Cheng, J. Li, C. Zhou, J. Lumin., 219, Article ID 116908 (2019).
Q. Wang, X.-M. Du, B. Zhao, M. L. Pang, Y. Li, and W.-J. Ruan, New J. Chem., 44, 1307–1312 (2020).
S. Megarajan and A. Veerappan, Opt. Mater., 108, Article ID 110177 (2020); https://doi.org/10.1016/j.optmat.2020.110177.
D. Anu, P. Naveen, R. Rajamanikandan, and M. V. Kaveri, J. Photochem. Photobiol. A: Chem., 405, Article ID 112921 (2021).
S. Rachel and G. Samantha, J. Chem. Edu., 98, 2643–2648 (2021).
A. Banerjee, A. Sahana, S. Das, S. Lohar, S. Guha, B. Sarkar, S. K. Mukhopadhyay, A. K. Mukherjee, and D. Das, Analyst, 137, 2166–2175 (2012).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Abstract of article is published in Zhurnal Prikladnoi Spektroskopii, Vol. 91, No. 3, p. 465, May–June, 2024.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cai, SQ., Zhang, KF. & Cai, XH. Highly Selective Fluorescent Sensor for Detection of Al3+ Based on Schiff-Base of 2-Hydroxy-1-Naphthaldehyde and 8-Aminoquinoline and Its Applications. J Appl Spectrosc (2024). https://doi.org/10.1007/s10812-024-01768-y
Published:
DOI: https://doi.org/10.1007/s10812-024-01768-y