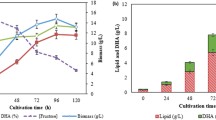
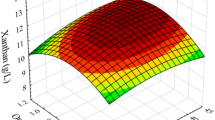
Abstract
A statistical experimental design using response surface methodology (RSM) was carried out to optimize the mixotrophic growth using glutamic acid, tri-sodium citrate, succinic acid, sodium aspartate, and sodium pyruvate to improve the production of fucoxanthin. First, a fractional factorial design as a screening test was done with the above-mentioned intermediates. Then, glutamic acid, sodium aspartate, and sodium pyruvate were chosen according to their statistically significant (P<0.05) and positive effects on fucoxanthin production. The three selected metabolic intermediates were optimized employing RSM to obtain a high level of fucoxanthin. The data were adjusted with a second-order polynomial equation to determine the relationship between fucoxanthin production and three optimized metabolic intermediates. Using multiple regression techniques and to attain the high fucoxanthin productivity (22.4 mg L−1 day−1), the optimum amount of the metabolic intermediates were found as follows: sodium aspartate, 7.5 mM; sodium pyruvate, 7.5 mM; glutamic acid, 3.29 mM.




Similar content being viewed by others
Availability of data and material
The data supporting the results of this study will be made available by the corresponding author upon reasonable request.
References
Alcantara S, Sanchez S (1999) Influence of carbon and nitrogen sources on Flavobacterium growth and zeaxanthin biosynthesis. J Ind Microbiol Biotechnol 23:697–700
Alkhamis Y, Qin JG (2016) Comparison of pigment and proximate compositions of Tisochrysis lutea in phototrophic and mixotrophic cultures. J Appl Phycol 28:35–42
Ausich RL (1997) Commercial opportunities for carotenoid production by biotechnology. Pure Appl Chem 69:2169–2174
Beuzenberg V, Goodwin EO, Puddick J, Romanazzi D, Adams SL, Packer MA (2017) Optimising conditions for growth and xanthophyll production in continuous culture of Tisochrysis lutea using photobioreactor arrays and central composite design experiments. N Z J Bot 55:64–78
Bhattacharjya R, Marella TK, Tiwari A, Saxena A, Singh PK, Mishra B (2020) Bioprospecting of marine diatoms Thalassiosira, Skeletonema and Chaetoceros for lipids and other value-added products. Bioresour Technol 318:124073
Bjork L (1969) Stimulation of β-carotene synthesis in Blakeslea trispora by pyruvate and intermediates of tricarboxylic acid (TCA) cycle. Acta Chem Scand 23:2908–2909
Chen T, Dong Wei Gu, Chen YW, Chen F (2009) Employment of organic acids to enhance astaxanthin formation in heterotrophic Chlorella zofingiensis. J Food Process Preserv 33:271–284
Chen J-H, Wei D, Lim P-E, **e J, Chen WN (2021) Screening and effect evaluation of chemical inducers for enhancing astaxanthin and lipid production in mixotrophic Chromochloris zofingiensis. J Appl Phycol 34:159–176
Choudhari SM, Ananthanarayan L, Singhal RS (2008) Use of metabolic stimulators and inhibitors for enhanced production of β-carotene and lycopene by Blakeslea trispora NRRL 2895 and 2896. Bioresour Technol 99:3166–3173
Cifuentes AS, Gonzalez MA, Vargas S, Hoeneisen M, Gonzalez N (2003) Optimization of biomass, total carotenoids and astaxanthin production in Haematococcus pluvialis Flotow strain Steptoe (Nevada, USA) under laboratory conditions. Biol Res 36:343–357
Czycan FC (1964) Canthaxanthin as secondary carotenoid in some green algae. Experientia 20:573–574
Davies KW (1993) Design of experiments for predictive microbial modeling. J Ind Microbiol 12:295–300
de Miguel T, Sieiro C, Poza M, Villa TG (2001) Analysis of canthaxanthin and related pigments from Gordonia jacobaea mutants. J Agric Food Chem 49:1200–1202
Doerfling P (1971) Significance of citric acid and other acids of the tricarboxylic acid cycle for growth and pigment formation in the unicellular alga Poteriochromonas stipitata. Z Allg Mikrobiol 11:161–165
Eilers U, Bikoulis A, Breitenbach J, Büchel C, Sandmann G (2016) Limitations in the biosynthesis of fucoxanthin as targets for genetic engineering in Phaeodactylum tricornutum. J Appl Phycol 28:123–129
Flores-Cotera L, Martin R, Sanchez S (2001) Citrate, a possible precursor of astaxanthin in Phaffia rhodozyma: influence of varying levels of ammonium, phosphate and citrate in a chemically defined medium. Appl Microbiol Biotechnol 55:341–347
Guler BA, Deniz I, Demirel Z, Oncel SS, Imamoglu E (2019) Transition from start-up to scale-up for fucoxanthin production in flat plate photobioreactor. J Appl Phycol 31:1525–1533
Harker M, Tsavalos AJ, Young AJ (1995) Use of response surface methodology to optimise carotenogenesis in the microalga Haematococcus pluvialis. J Appl Phycol 7:399–406
Ishika T, Laird DW, Bahri PA, Moheimani NR (2019) Co-cultivation and stepwise cultivation of Chaetoceros muelleri and Amphora sp. for fucoxanthin production under gradual salinity increase. J Appl Phycol 31:1535–1544
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz 167:191–194
Kim SM, Kang S-W, Kwon O-N, Chung D, Pan C-H (2012) Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: characterization of extraction for commercial application. J Korean Soc Appl Biol Chem 55:477–483
Kobayashi M, Kakizono T, Nagai S (1991) Astaxanthin production by a green alga, Haematococcus pluvialis accompanied with morphological changes in acetate media. J Ferment Bioeng 71:335–339
Li H-B, Fan K-W, Chen F (2006) Isolation and purification of canthaxanthin from the microalga Chlorella zofingiensis by high-speed counter-current chromatography. J Sep Sci 29:699–703
Liu J, Sun Z, Zhong Y, Gerken H, Huang J, Chen F (2013) Utilization of cane molasses towards cost-saving astaxanthin production by a Chlorella zofingiensis mutant. J Appl Phycol 25:1447–1456
Markou G, Nerantzis E (2013) Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol Adv 31:1532–1542
Mohamadnia S, Tavakoli O, Faramarzi MA, Shamsollahi Z (2020b) Production of fucoxanthin by the microalga Tisochrysis lutea: a review of recent developments. Aquaculture 516:734637
Mohamadnia S, Tavakoli O, Faramarzi MA (2021) Enhancing production of fucoxanthin by the optimization of culture media of the microalga Tisochrysis lutea. Aquaculture 533:736074
Myers RH, Montgomery DC, Anderson-Cook CM (2002) Response surface methodology: process and product optimization using designed experiments. Wiley, NY
Nasrabadi MRN, Razavi SH (2010) Use of response surface methodology in a fed-batch process for optimization of tricarboxylic acid cycle intermediates to achieve high levels of canthaxanthin from Dietzia natronolimnaea HS-1. J Biosci Bioeng 109:361–368
Nefelova MV, Sverdlova AN, Alekseeva LN (1978) Effect of organic acids on the biosynthesis of carotenes by an Actinomyces chrysomallus strain. Mikrobiologiia 47:208–211
Pelah D, Sintov A, Cohen E (2004) The effect of salt stress on the production of canthaxanthin and astaxanthin by Chlorella zofingiensis grown under limited light intensity. World J Microbiol Biotechnol 20:483–486
Razavi SH, Marc I (2006) Effect of temperature and pH on the growth kinetics and carotenoid production by Sporobolomyces ruberrimus H110 using technical glycerol as carbon source. Iran J Chem Chem Eng 25:59–64
Sahin MS, Khazi MI, Demirel Z, Dalay MC (2019) Variation in growth, fucoxanthin, fatty acids profile and lipid content of marine diatoms Nitzschia sp. and Nanofrustulum shiloi in response to nitrogen and iron. Biocatal Agric Biotechnol 17:390–398
Sandmann G (1994) Carotenoid biosynthesis in microorganisms and plants. Eur J Biochem 223:7–24
**a S, Ke W, Wan L, Li A, Qiang Hu, Zhang C (2013) Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar Drugs 11:2667–2681
**ong C, Shouwen C, Ming S, Ziniu Yu (2005) Medium optimization by response surface methodology for poly-γ-glutamic acid production using dairy manure as the basis of a solid substrate. Appl Microbiol Biotechnol 69:390–396
Zhang D-H, Lee Y-K (2001) Two-step process for ketocarotenoid production by a green alga, Chlorococcum sp. strain MA-1. Appl Microbiol Biotechnol 55:537–540
Zhang L, Zhang C, Liu J, Yang N (2020) A strategy for stimulating astaxanthin and lipid production in Haematococcus pluvialis by exogenous glycerol application under low light. Algal Res 46:101779
Author information
Authors and Affiliations
Contributions
Sonia Mohamadnia designed the study and experiments. Sonia Mohamadnia performed the experiments and data analysis. Sonia Mohamadnia drafted the manuscript. Omid Tavakoli and Mohammad Ali Faramarzi reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohamadnia, S., Tavakoli, O. & Faramarzi, M.A. Optimization of metabolic intermediates to enhance the production of fucoxanthin from Tisochrysis lutea. J Appl Phycol 34, 1269–1279 (2022). https://doi.org/10.1007/s10811-022-02717-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02717-y