Abstract
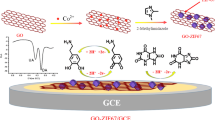
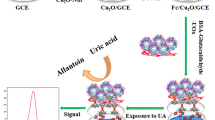
UiO-66 based materials (University of Oslo-66), with large surface area and abundant active sites, exhibit high water/solution stability due to a strong interaction between zirconium ion and the oxygen atom in carboxylate linkers. Therefore, they are potential electrode modifiers for electrochemical analysis because most of the electrochemical sensors have been used in aqueous media. In the present study, a potential electrochemical technique to detect uric acid (URA) and xanthine (XAN) is described using a Co/UiO-66 modified glassy carbon electrode. The Co/UiO-66 materials with different Co/Zr molar ratios were synthesized by hydrothermal process and were characterized by X-ray diffraction, scanning electron microscopy, X-ray photoelectron spectroscopy, and nitrogen adsorption/desorption isotherms. The characterization showed a low loading and high dispersion of doped Co onto UiO-66 matrix. It was also found that the doped Co element may be grafted into the lattice of UiO-66. Co/UiO-66 exhibited better electrochemical activity toward URA and XAN detection. Under optimum conditions, 0.08Co/UiO-66/GCE demonstrated the best response compared to other material of different Co/Zr ratio, with three linear calibration ranges of 5–10 µM, 10-49.5 µM, and 49.5–327.1 µM and the limit of detection of 0.544 and 0.547 µM for URA and XAN, respectively. This proposed sensor offers a simple and low-cost pathway to quantify URA and XAN and was successfully employed to simultaneously determine URA and XAN in human urine with exceptional recoveries (97.61 to 104.79%).
Graphical abstract











Similar content being viewed by others
References
Yamamoto T, Moriwaki Y, Takahashi S (2005) Effect of ethanol on metabolism of purine bases (hypoxanthine, xanthine, and uric acid). Clin Chim Acta 356:35–57. https://doi.org/10.1016/j.cccn.2005.01.024
Jesny S, Girish Kumar K (2017) Non-enzymatic electrochemical sensor for the simultaneous determination of xanthine, its methyl derivatives theophylline and caffeine as well as its metabolite uric acid. Electroanalysis 29:1828–1837. https://doi.org/10.1002/elan.201700115
Krakoff IH (1965) Studies of uric acid biosynthesis in the chronic leukemias. Arthritis Rheum 8:772–779. https://doi.org/10.1002/art.1780080440
Vernerová A, Krčmová LK, Heneberk O et al (2021) Chromatographic method for the determination of inflammatory biomarkers and uric acid in human saliva. Talanta 233:122598. https://doi.org/10.1016/j.talanta.2021.122598
Tan H, Li Y (2021) AIEgens-based fluorescent covalent organic framework in construction of chemiluminescence resonance energy transfer system for serum uric acid detection. Microchim Acta 188:254. https://doi.org/10.1007/s00604-021-04923-w
Mu G, Luan F, Xu L et al (2012) Determination of purines in soybean milk by capillary electrophoresis in comparison with high performance liquid chromatography. Anal Methods 4:3386–3391. https://doi.org/10.1039/C2AY25488C
Guo Z, Wang G, Li J, Wu D, Guo X (2021) A miniaturized electrochemical biosensor based on poly(l- threonine) modified pencil graphite electrodes and its application for trace-level determination of uric acid, xanthine and hypoxanthine. Int J Electrochem Sci 16:210262. https://doi.org/10.20964/2021.01.05
Ndlovu T, Arotiba OA, Sampath S et al (2012) An exfoliated graphite-based bisphenol a electrochemical sensor. Sensors 12:11601–11611. https://doi.org/10.3390/s120911601
Ghanbari MH, Mashhadizadeh MH, Norouzi Z, Salehzadeh H (2022) Simultaneous electrochemical detection of uric acid and xanthine based on electrodeposited B, N co-doped reduced graphene oxide, gold nanoparticles and electropolymerized poly (L-cysteine) gradually modified electrode platform. Microchem J 175:107213. https://doi.org/10.1016/j.microc.2022.107213
Ganesan M, Ramadhass KD, Chuang H-C, Gopalakrishnan G (2021) Synthesis of nitrogen-doped carbon quantum dots@Fe2O3/multiwall carbon nanotubes ternary nanocomposite for the simultaneous electrochemical detection of 5-fluorouracil, uric acid, and xanthine. J Mol Liq 331:115768. https://doi.org/10.1016/j.molliq.2021.115768
Tang T, Zhou M, Lv J et al (2022) Sensitive and selective electrochemical determination of uric acid in urine based on ultrasmall iron oxide nanoparticles decorated urchin-like nitrogen-doped carbon. Colloids Surf B Biointerfaces 216:112538. https://doi.org/10.1016/j.colsurfb.2022.112538
Zhu D, **n J, Li X (2022) Self-assembly encapsulation of vanadium tetrasulfide into nitrogen doped biomass-derived porous carbon as a high performance electrochemical sensor for xanthine determination. New J Chem 46:12773–12782. https://doi.org/10.1039/D2NJ02113G
Amini A, Kazemi S, Safarifard V (2020) Metal-organic framework-based nanocomposites for sensing applications-a review. Polyhedron 177:114260. https://doi.org/10.1016/j.poly.2019.114260
Wei N, Zhang M-Y, Zhang X-N et al (2014) Two series of solvent-dependent lanthanide coordination polymers demonstrating tunable luminescence and catalysis properties. Cryst Growth Des 14:3002–3009. https://doi.org/10.1021/cg500286v
Cavka JH, Jakobsen S, Olsbye U et al (2008) A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J Am Chem Soc 130:13850–13851. https://doi.org/10.1021/ja8057953
Kar AK, Sarkar R, Manal AK et al (2023) Unveiling and understanding the remarkable enhancement in the catalytic activity by the defect creation in UIO-66 during the catalytic transfer hydrodeoxygenation of vanillin with isopropanol. Appl Catal B Environ 325:122385. https://doi.org/10.1016/j.apcatb.2023.122385
Mirzaei K, Jafarpour E, Shojaei A et al (2023) An investigation on the influence of highly acidic media on the microstructural stability and dye adsorption performance of UiO-66. Appl Surf Sci 618:156531. https://doi.org/10.1016/j.apsusc.2023.156531
Pourmadadi M, Omrani Z, Forootan Z et al (2023) UiO-66 nanoparticles as a drug delivery system: a comprehensive review. J Drug Deliv Sci Technol 86:104690. https://doi.org/10.1016/j.jddst.2023.104690
Wang Y, Lu Y, Hu Z et al (2023) Facile preparation of Zr@carbon electrodes based on polyimide/UiO-66 composites for supercapacitors. Electrochem Commun 148:107449. https://doi.org/10.1016/j.elecom.2023.107449
Zhang H, **ong P, Li G et al (2020) Applications of multifunctional zirconium-based metal-organic frameworks in analytical chemistry: overview and perspectives. TrAC Trends Anal Chem 131:116015. https://doi.org/10.1016/j.trac.2020.116015
Chang T-E, Chuang C-H, Kung C-W (2021) An iridium-decorated metal–organic framework for electrocatalytic oxidation of Nitrite. Electrochem Commun 122:106899. https://doi.org/10.1016/j.elecom.2020.106899
Chuang C-H, Kung C-W (2020) Metal – organic frameworks toward electrochemical sensors: challenges and opportunities. Electroanalysis 32:1885–1895. https://doi.org/10.1002/elan.202060111
Ding Y, Wei F, Dong C et al (2021) UiO-66 based electrochemical sensor for simultaneous detection of cd(II) and pb(II). Inorg Chem Commun 131:108785. https://doi.org/10.1016/j.inoche.2021.108785
Li Y, Shen Y, Zhang Y et al (2021) A UiO-66-NH2/carbon nanotube nanocomposite for simultaneous sensing of dopamine and acetaminophen. Anal Chim Acta 1158:338419. https://doi.org/10.1016/j.aca.2021.338419
Britton HTS, Robinson RA (1931) CXCVIII—universal buffer solutions and the dissociation constant of veronal. J Chem Soc. https://doi.org/10.1039/JR9310001456
Ahmed M, Al-Hadeethi YM, Alshahrie A et al (2022) The role of cobalt to control the synthesis of nanoscale Co/UiO-66 composite for photocatalysis. J Am Ceram Soc 105:7043–7052. https://doi.org/10.1111/jace.18672
Nhi LTT, Tu NTT, Hoa LT et al (2021) Simultaneous voltammetric determination of ascorbic acid and acetaminophen in pharmaceutical formulations with UiO-66-modified glassy carbon electrode. J Nanoparticle Res 23:218. https://doi.org/10.1007/s11051-021-05327-w
Shearer GC, Chavan S, Bordiga S et al (2016) Defect engineering: yuning the porosity and composition of the metal–organic framework UiO-66 via modulated synthesis. Chem Mater 28:3749–3761. https://doi.org/10.1021/acs.chemmater.6b00602
Wang Y-S, Liao J-L, Li Y-S et al (2020) Zirconium-based metal–organic framework nanocomposites containing dimensionally distinct nanocarbons for pseudocapacitors. ACS Appl Nano Mater 3:1448–1456. https://doi.org/10.1021/acsanm.9b02297
Wang Y-S, Chen Y-C, Li J-H, Kung C-W (2019) Toward metal–organic-framework-based supercapacitors: room-temperature synthesis of electrically conducting mof-based nanocomposites decorated with redox-active manganese. Eur J Inorg Chem 2019:3036–3044. https://doi.org/10.1002/ejic.201900584
Hu J, Liu Y, Liu J et al (2018) High CO2 adsorption capacities in UiO type MOFs comprising heterocyclic ligand. Microporous Mesoporous Mater 256:25–31. https://doi.org/10.1016/j.micromeso.2017.07.051
Li J, Wang S, **ao T et al (2017) Controllable preparation of nanoporous Ni3S2 films by sulfuration of nickel foam as promising asymmetric supercapacitor electrodes. Appl Surf Sci 420:919–926. https://doi.org/10.1016/j.apsusc.2017.05.206
Shaaban ER (2013) Optical constants and fitted transmittance spectra of varies thickness of polycrystalline ZnSe thin films in terms of spectroscopic ellipsometry. J Alloys Compd 563:274–279. https://doi.org/10.1016/j.jallcom.2013.02.132
Zheng J, Lei Z (2018) Incorporation of CoO nanoparticles in 3D marigold flower-like hierarchical architecture MnCo2O4 for highly boosting solar light photo-oxidation and reduction ability. Appl Catal B Environ 237:1–8. https://doi.org/10.1016/j.apcatb.2018.05.060
Deng P, Xu Z, Zeng R, Ding C (2015) Electrochemical behavior and voltammetric determination of vanillin based on an acetylene black paste electrode modified with graphene–polyvinylpyrrolidone composite film. Food Chem 180:156–163. https://doi.org/10.1016/j.foodchem.2015.02.035
Bard AJ, Faulkner LR (2001) Fundamentals and applications: electrochemical methods, 2nd edn. Wiley, Hoboken
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem 101:19–28. https://doi.org/10.1016/S0022-0728(79)80075-3
Dryhurst G (1972) Electrochemical oxidation of uric acid and xanthine at the pyrolytic graphite electrode: mechanistic interpretation of electrochemistry. J Electrochem Soc 119:1659. https://doi.org/10.1149/1.2404066
Kumar AS, Swetha P (2010) Ru(DMSO)4Cl2 nano-aggregated nafion membrane modified electrode for simultaneous electrochemical detection of hypoxanthine, xanthine and uric acid. J Electroanal Chem 642:135–142. https://doi.org/10.1016/j.jelechem.2010.02.031
Kalimuthu P, John SA (2010) Simultaneous determination of ascorbic acid, dopamine, uric acid and xanthine using a nanostructured polymer film modified electrode. Talanta 80:1686–1691. https://doi.org/10.1016/j.talanta.2009.10.007
Sun D, Zhang Y, Wang F et al (2009) Electrochemical sensor for simultaneous detection of ascorbic acid, uric acid and xanthine based on the surface enhancement effect of mesoporous silica. Sens Actuators B Chem 141:641–645. https://doi.org/10.1016/j.snb.2009.07.043
Ibrahim H, Temerk Y (2016) Sensitive electrochemical sensor for simultaneous determination of uric acid and xanthine in human biological fluids based on the nano-boron doped ceria modified glassy carbon paste electrode. J Electroanal Chem 780:176–186. https://doi.org/10.1016/j.jelechem.2016.09.016
Sen S, Sarkar P (2020) A simple electrochemical approach to fabricate functionalized MWCNT-nanogold decorated PEDOT nanohybrid for simultaneous quantification of uric acid, xanthine and hypoxanthine. Anal Chim Acta 1114:15–28. https://doi.org/10.1016/j.aca.2020.03.060
Acknowledgements
This study is funded by the Ministry of Education and Training - Vietnam (No B2022- DNA-16).
Author information
Authors and Affiliations
Contributions
VTN: conceptualization, validation. NQM: methodology, investigation. PTHT: data curation. DQK and TDM: writing—review & editing. TDM : supervision. formal analysis. VTN: resources.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nguyen, V.T., Manh, T.D., Man, N.Q. et al. Electrochemical detection of uric acid and xanthine in human urine using the Co/UiO-66 modified glassy carbon electrode. J Appl Electrochem (2024). https://doi.org/10.1007/s10800-024-02102-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10800-024-02102-2