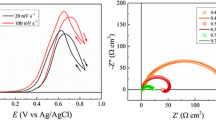
Abstract
Glycerol is a by-product obtained in the transesterification reaction together with biodiesel, a necessary renewable fuel. The presence of glycerol in fuel can bring up environmental harm and engine damage, making fuel quality control important. In this context, composite electrodes (CE) were developed with low-cost materials like syringes, copper wire, graphite, and paraffin. The surface of composite electrodes was modified with reduced graphene oxide (RGO) and hydroxide nickel (Ni(OH)2). SEM, EDX, Raman, and FT-IR ATR characterized the developed material. The electrooxidation of glycerol in an alkaline medium containing 0.10 M of NaOH was investigated by CV. In the presence of glycerol, the modified electrode increased the anodic and cathodic peak current with an anodic peak potential shift to 0.54 V. The electrodes were employed in a study of the determination of glycerol in real biodiesel samples by a chronoamperometric technique. The modified electrode showed a sensitivity of 1.63 × 103 µA mM and a LOD for glycerol of 2.24 × 10–5 M (R = 0.996). The mean response time of the modified electrodes to glycerol was 1.85 s. These electrodes show good reproducibility and competitive detection limit compared to the literature. The proposed method presented satisfactory results, with 94.98 and 100.39% sample recoveries.
Graphical abstract











Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in the published article.
References
Empresa de Pesquisa Energética (2021) Balanço Nacional de Energia
Farahani VJ, Pirhadi M, Sioutas C (2021) Are standardized diesel exhaust particles (DEP) representative of ambient particles in air pollution toxicological studies? Sci Total Environ 788:147854. https://doi.org/10.1016/j.scitotenv.2021.147854
Infante EG, Secchi AR, Leite LF, Souza Antunes AM (2021) Scientific articles and patent applications on biodiesel production from lignocellulosic biomass. J Environ Prot (Irvine, Calif) 12:371–390. https://doi.org/10.4236/jep.2021.126023
Lourenço LM, Stradiotto NR (2009) Determination of free glycerol in biodiesel at a platinum oxide surface using potential cycling technique. Talanta 79:92–96. https://doi.org/10.1016/j.talanta.2009.03.013
Tehrani RMA, Ab Ghani S (2012) Electrocatalysis of free glycerol at a nanonickel modified graphite electrode and its determination in biodiesel. Electrochim Acta 70:153–157. https://doi.org/10.1016/j.electacta.2012.03.044
Cazumbá A, Cunha F, Silva MT, Paiva T (2022) Evaluation of production processes of glycerol acetals using process intensification by flow chemistry. Chem Eng Process Process Intensif 177:108997. https://doi.org/10.1016/j.cep.2022.108997
Kaur J, Sarma AK, Jha MK, Gera P (2020) Valorisation of crude glycerol to value-added products: perspectives of process technology, economics and environmental issues. Biotechnol Rep 27:e00487. https://doi.org/10.1016/j.btre.2020.e00487
Ladero M (2021) New Glycerol Upgrading Processes. Catalysts 11:103. https://doi.org/10.3390/catal11010103
Gordhan D, Swainson SME, Pearce AK et al (2020) Poly (glycerol adipate): from a functionalized nanocarrier to a polymeric-prodrug matrix to create amorphous solid dispersions. J Pharm Sci 109:1347–1355. https://doi.org/10.1016/j.xphs.2019.12.004
Dodekatos G, Schünemann S, Tüysüz H (2018) Recent advances in thermo-, photo-, and electrocatalytic glycerol oxidation. ACS Catal 8:6301–6333. https://doi.org/10.1021/acscatal.8b01317
Díaz-Lasprilla AM, Mercado RA, Ramírez-Caballero GE (2021) Glycerol polymerization degree effect on the emulsifying properties of polyglycerol esters. J Appl Polym Sci. https://doi.org/10.1002/app.50566
Leal-Duaso A, Favier I, Pla D et al (2021) Design of glycerol-based solvents for the immobilization of palladium nanocatalysts: a hydrogenation study. ACS Sustain Chem Eng 9:6875–6885. https://doi.org/10.1021/acssuschemeng.1c01694
Brza MA, AzizAnuar BS et al (2020) The study of EDLC device with high electrochemical performance fabricated from proton ion conducting PVA-based polymer composite electrolytes plasticized with glycerol. Polymers (Basel) 12:1896. https://doi.org/10.3390/polym12091896
Soares Dias AP, Gomes Fonseca F, Catarino M, Gomes J (2021) Biodiesel glycerin valorization into oxygenated fuel additives. Catal Lett 152:513
Arévalo FJ, Osuna-Sánchez Y, Sandoval-Cortés J et al (2017) Development of an electrochemical sensor for the determination of glycerol based on glassy carbon electrodes modified with a copper oxide nanoparticles/multiwalled carbon nanotubes/pectin composite. Sens Actuators B Chem 244:949–957. https://doi.org/10.1016/j.snb.2017.01.093
Gerhard K, Krahl J (2015) The biodiesel handbook, 2nd edn. Elsevier Science, Champaign
dos Assis KLSC, Archanjo BS, Achete CA (2020) A new sensor based on reduced graphene oxide/Au nanoparticles for glycerol detection. Mater Res. https://doi.org/10.1590/1980-5373-mr-2019-0513
Chen WC, Li PY, Chou CH et al (2015) A nonenzymatic approach for selective and sensitive determination of glycerol in biodiesel based on a PtRu-modified screen-printed edge band ultramicroelectrode. Electrochim Acta 153:295–299. https://doi.org/10.1016/j.electacta.2014.12.011
Ribeiro MS, Rocha FRP (2013) A single-phase spectrophotometric procedure for in situ analysis of free glycerol in biodiesel. Microchem J 106:23–26. https://doi.org/10.1016/j.microc.2012.04.011
Born A, Foged L, Van Hall G (2014) Glucose and glycerol concentrations and their tracer enrichment measurements using liquid chromatography tandem mass spectrometry. J Mass Spectrom 49:980–988. https://doi.org/10.1002/jms.3407
Ramonas E, Ratautas D, Dagys M et al (2019) Highly sensitive amperometric biosensor based on alcohol dehydrogenase for determination of glycerol in human urine. Talanta 200:333–339. https://doi.org/10.1016/j.talanta.2019.03.063
Ghoreishi SM, Khoobi A, Behpour M, Masoum S (2014) Application of multivariate curve resolution alternating least squares to biomedical analysis using electrochemical techniques at a nanostructure-based modified sensor. Electrochim Acta 130:271–278. https://doi.org/10.1016/j.electacta.2014.03.016
Shaterian M, Khoobi A, Enhessari M, Ozaee K (2015) A new strategy based on thermodiffusion of ceramic nanopigments into metal surfaces and formation of anti-corrosion coatings. Microporous Mesoporous Mater 218:62–68. https://doi.org/10.1016/j.micromeso.2015.06.039
Heydari M, Ghoreishi SM, Khoobi A (2019) Novel electrochemical procedure for sensitive determination of Sudan II based on nanostructured modified electrode and multivariate optimization. Measurement 142:105–112. https://doi.org/10.1016/j.measurement.2019.04.058
Ghoreishi SM, Behpour M, Khoobi A, Moghadam Z (2013) Determination of trace amounts of sulfamethizole using a multi-walled carbon nanotube modified electrode: application of experimental design in voltammetric studies. Anal Lett 46:323–339. https://doi.org/10.1080/00032719.2012.718831
Ghoreishi SM, Kashani FZ, Khoobi A, Enhessari M (2015) Fabrication of a nickel titanate nanoceramic modified electrode for electrochemical studies and detection of salicylic acid. J Mol Liq 211:970–980. https://doi.org/10.1016/j.molliq.2015.08.035
Cazula BB, Lazarin AM (2017) Development of chemically modified carbon paste electrodes with transition metal complexes anchored on silica gel. Mater Chem Phys 186:470–477. https://doi.org/10.1016/j.matchemphys.2016.11.021
Shabalina AV, Svetlichnyi VA, Ryzhinskaya KA, Lapin IN (2017) Copper nanoparticles for ascorbic acid sensing in water on carbon screen-printed electrodes. Anal Sci 33:1415–1419. https://doi.org/10.2116/analsci.33.1415
Yang X, Wang L, Zhou G et al (2015) Electrochemical detection of H2O2 based on Fe3O4 nanoparticles with graphene oxide and polyamidoamine dendrimer. J Clust Sci 26:789–798. https://doi.org/10.1007/s10876-014-0746-9
Đurović A, Stojanović Z, Bytešníková Z et al (2022) Reduced graphene oxide/ZnO nanocomposite modified electrode for the detection of tetracycline. J Mater Sci 57:5533–5551. https://doi.org/10.1007/s10853-022-06926-1
Ahmadi E, Zarei E, Asghari A (2023) Electrochemical sensor based on graphene and tungsten disulfide nanoparticles for determination of noscapine and papaverine. Ionics (Kiel) 29:1579–1591. https://doi.org/10.1007/s11581-023-04895-2
Bounegru AV, Apetrei C (2020) Carbonaceous nanomaterials employed in the development of electrochemical sensors based on screen-printing technique—a review. Catalysts 10:680. https://doi.org/10.3390/catal10060680
Albright TB, Hobeck JD (2020) Development of manufacturing and characterization methods for carbon black-based conductive polymer composite sensors. Advanced materials: design, processing, characterization, and applications. American Society of Mechanical Engineers
Chimezie AB, Hajian R, Yusof NA et al (2017) Fabrication of reduced graphene oxide-magnetic nanocomposite (rGO-Fe3O4) as an electrochemical sensor for trace determination of As(III) in water resources. J Electroanal Chem 796:33–42. https://doi.org/10.1016/j.jelechem.2017.04.061
Paiva VM, dos Assis KL et al (2021) Electrochemical analysis of free glycerol in biodiesel using reduced graphene oxide and gold/palladium core-shell nanoparticles modified glassy carbon electrode. Processes 9:1389. https://doi.org/10.3390/pr9081389
Oliveira JPJ, Vilalva JB, de Sá AC, Paim LL (2020) Electrosynthesis of composites consisting of FeOOH and reduced graphene oxide in graphite electrodes. Orbital Electron J Chem. https://doi.org/10.17807/orbital.v12i3.1477
Sousa MSP, de Sá AC, de Oliveira JPJ et al (2020) Impedimetric sensor for pentoses based on electrodeposited carbon nanotubes and molecularly imprinted poly-o-phenylenediamine. ECS J Solid State Sci Technol 9:041006. https://doi.org/10.1149/2162-8777/ab85bd
de Oliveira JPJ, de Sá AC, Sousa MSP et al (2021) A facile controlled-synthesis method of nanoparticles of nickel oxide/hydroxide anchored in graphite/RGO for alcohol oxidation. ECS J Solid State Sci Technol 10:011001. https://doi.org/10.1149/2162-8777/abdc42
Chen L, Tang Y, Wang K et al (2011) Direct electrodeposition of reduced graphene oxide on glassy carbon electrode and its electrochemical application. Electrochem commun 13:133–137. https://doi.org/10.1016/j.elecom.2010.11.033
de Oliveira JPJ, Hiranobe CT, Torres GB et al (2022) Determination of Cr(VI) in leather residues using graphite/paraffin composite electrodes modified with reduced graphene oxide nanosheets. Sci Total Environ 834:155318. https://doi.org/10.1016/j.scitotenv.2022.155318
de Sá AC, Paim LL, Stradiotto NR (2014) Sugars electrooxidation at glassy carbon electrode decorate with multi-walled carbon nanotubes with nickel oxy-hydroxide. Int J Electrochem Sci 9:7746–7762
Ballottin DPM, Paim LL, Stradiotto NR (2013) Determination of glycerol in biodiesel using a nickel(II) oxyhydroxide chemically modified electrode by cyclic voltammetry. Electroanalysis 25:1751–1755. https://doi.org/10.1002/elan.201300090
de Oliveira JPJ, Emeterio MBS, de Sá AC et al (2019) Methanol, ethanol, and glycerol oxidation by graphite-epoxy composite electrodes with graphene-anchored nickel oxyhydroxide nanoparticles. MDPI, Basel Switzerland, p 5
Sedenho GC, Paim LL, Stradiotto NR et al (2013) Simple and direct potentiometric determination of potassium ions in biodiesel microemulsions at a glassy carbon electrode modified with nickel(ii) hexacyanoferrate nanoparticles. Anal Methods 5:4145. https://doi.org/10.1039/c3ay40774h
Cataldi TRI, Guascito R, Salvi AM (1996) XPS study and electrochemical behaviour of the nickel hexacyanoferrate film electrode upon treatment in alkaline solutions. J Electroanal Chem 417:83–88. https://doi.org/10.1016/S0022-0728(96)04749-3
Trafela Š, Zavašnik J, Šturm S, Žužek Rožman K (2020) Controllable voltammetric formation of a structurally disordered NiOOH/Ni(OH)2 redox pair on Ni-nanowire electrodes for enhanced electrocatalytic formaldehyde oxidation. Electrochim Acta 362:137180. https://doi.org/10.1016/j.electacta.2020.137180
Houache MSE, Cossar E, Ntais S, Baranova EA (2018) Electrochemical modification of nickel surfaces for efficient glycerol electrooxidation. J Power Sources 375:310–319. https://doi.org/10.1016/j.jpowsour.2017.08.089
He L, Zhou W, Cai D et al (2017) Pulsed laser-deposited n-Si/NiOx photoanodes for stable and efficient photoelectrochemical water splitting. Catal Sci Technol 7:2632–2638. https://doi.org/10.1039/C7CY00114B
Wang F, Feng Y, He S et al (2020) Nickel nanoparticles-loaded three-dimensional porous magnetic graphene-like material for non-enzymatic glucose sensing. Microchem J 155:104748. https://doi.org/10.1016/j.microc.2020.104748
da Silva JL, Beluomini MA, Sedenho GC, Stradiotto NR (2017) Determination of amino acids in sugarcane vinasse by ion chromatographic using nickel nanoparticles on reduced graphene oxide modified electrode. Microchem J 134:374–382. https://doi.org/10.1016/j.microc.2017.07.007
Wang R, Liu H, Zhang K et al (2021) Ni(II)/Ni(III) redox couple endows Ni foam-supported Ni2P with excellent capability for direct ammonia oxidation. Chem Eng J 404:126795. https://doi.org/10.1016/j.cej.2020.126795
Alinejadian N, Kazemi SH, Nasirpouri F, Odnevall I (2023) Electro-deposited nano-Ni/reduced graphene oxide composite film of corrugated surface for high voltammetric sensitivity. Mater Chem Phys 297:127288. https://doi.org/10.1016/j.matchemphys.2022.127288
Rahmani K, Habibi B (2020) Electrofabrication of the ternary NiCuFe alloy nanoparticles/ERGO nanocomposite: effective electrooxidation of the glucose and glycerol in alkaline media. ChemistrySelect 5:7990–8001. https://doi.org/10.1002/slct.202001561
Tamiji T, Nezamzadeh-Ejhieh A (2019) Electrocatalytic behavior of AgBr NPs as modifier of carbon past electrode in the presence of methanol and ethanol in aqueous solution: a kinetic study. J Taiwan Inst Chem Eng 104:130–138. https://doi.org/10.1016/j.jtice.2019.08.021
Pillai R, Preetha S, Narasimhamurthy B, Lekshmi IC (2022) Biosensing of catechol via amperometry using laccase immobilized nickel oxide/graphite modified screen-printed electrodes. Mater Today Proc 62:5434–5438. https://doi.org/10.1016/j.matpr.2022.03.708
da Silva JL, Buffon E, Beluomini MA et al (2021) Non-enzymatic lactose molecularly imprinted sensor based on disposable graphite paper electrode. Anal Chim Acta 1143:53–64. https://doi.org/10.1016/j.aca.2020.11.030
Alves GM, da Silva JL, Stradiotto NR (2021) A novel citrus pectin-modified carbon paste electrochemical sensor used for copper determination in biofuel. Measurement. https://doi.org/10.1016/j.measurement.2020.108356
Jagiełło J, Chlanda A, Baran M et al (2020) Synthesis and characterization of graphene oxide and reduced graphene oxide composites with inorganic nanoparticles for biomedical applications. Nanomaterials 10:1846. https://doi.org/10.3390/nano10091846
Mukhortova YR, Pryadko AS, Chernozem RV et al (2022) Fabrication and characterization of a magnetic biocomposite of magnetite nanoparticles and reduced graphene oxide for biomedical applications. Nano-Struct Nano-Objects 29:100843. https://doi.org/10.1016/j.nanoso.2022.100843
Chen Y, Huang Z, Zhang H et al (2014) Synthesis of the graphene/nickel oxide composite and its electrochemical performance for supercapacitors. Int J Hydrogen Energy 39:16171–16178. https://doi.org/10.1016/j.ijhydene.2014.01.165
Quezada-Renteria JA, Ania CO, Chazaro-Ruiz LF, Rangel-Mendez JR (2019) Influence of protons on reduction degree and defect formation in electrochemically reduced graphene oxide. Carbon NY 149:722–732. https://doi.org/10.1016/j.carbon.2019.04.109
Izquierdo J, Mizaikoff B, Kranz C (2016) Surface-enhanced infrared spectroscopy on boron-doped diamond modified with gold nanoparticles for spectroelectrochemical analysis. Phys Status Solidi (a) 213:2056–2062. https://doi.org/10.1002/pssa.201600222
Mavis B, Akinc M (2006) Cyanate intercalation in nickel hydroxide. Chem Mater 18:5317–5325. https://doi.org/10.1021/cm0528835
Yao J, Huang R, Li Y et al (2023) Facile hydrothermal fabrication of an α-Ni(OH)2/N-doped reduced graphene oxide nanohybrid as a high-performance anode material for lithium-ion batteries. Energy Fuels. https://doi.org/10.1021/acs.energyfuels.2c03341
Guo H, Wang X, Qian Q et al (2009) A green approach to the synthesis of graphene nanosheets. ACS Nano 3:2653–2659. https://doi.org/10.1021/nn900227d
Mirzaee M, Dehghanian C, Sarbishei S (2018) Facile synthesis of nano dendrite-structured Ni–NiO foam/ERGO by constant current method for supercapacitor applications. J Appl Electrochem 48:923–935. https://doi.org/10.1007/s10800-018-1229-8
Mekassa B, Baker PGL, Chandravanshi BS, Tessema M (2018) Synthesis, characterization, and preparation of nickel nanoparticles decorated electrochemically reduced graphene oxide modified electrode for electrochemical sensing of diclofenac. J Solid State Electrochem 22:3607–3619. https://doi.org/10.1007/s10008-018-4071-3
Ghaith ME, Abd El-Moghny MG, El-Nagar GA et al (2023) Tailor-designed binary Ni–Cu nano dendrites decorated 3D-carbon felts for efficient glycerol electrooxidation. RSC Adv 13:895–905. https://doi.org/10.1039/D2RA06853B
Wang H, Ding J, Kannan P et al (2020) Nitrogen-doped mesoporous carbon nanosheet network entrapped nickel nanoparticles as an efficient catalyst for electro-oxidation of glycerol. Int J Hydrogen Energy 45:28821–28835. https://doi.org/10.1016/j.ijhydene.2020.07.269
Antolini E (2019) Glycerol electro-oxidation in alkaline media and alkaline direct glycerol fuel cells. Catalysts 9:980. https://doi.org/10.3390/catal9120980
Houache MSE, Sandoval MG, Safari R et al (2021) Morphology alteration of nickel microstructures for glycerol electrooxidation. J Catal 404:348–361. https://doi.org/10.1016/j.jcat.2021.10.010
Oliveira VL, Morais C, Servat K et al (2013) Glycerol oxidation on nickel based nanocatalysts in alkaline medium—Identification of the reaction products. J Electroanal Chem 703:56–62. https://doi.org/10.1016/j.jelechem.2013.05.021
Huang L, Sun JY, Cao SH et al (2016) Combined EC-NMR and in situ FTIR spectroscopic studies of glycerol electrooxidation on Pt/C, PtRu/C, and PtRh/C. ACS Catal 6:7686–7695. https://doi.org/10.1021/acscatal.6b02097
Ghosh P, Chaudhari PK, Thakur C (2022) Advances in chemical and environmental engineering (ACEE). Environ Sci Pollut Res 29:86374–86375. https://doi.org/10.1007/s11356-022-23783-2
Bott-Neto JL, Martins TS, Machado SAS, Ticianelli EA (2019) Electrocatalytic oxidation of methanol, ethanol, and glycerol on Ni(OH)2 nanoparticles encapsulated with poly[Ni( salen )] film. ACS Appl Mater Interfaces 11:30810–30818. https://doi.org/10.1021/acsami.9b08441
Kurt Urhan B, Öztürk Doğan H, Çepni E et al (2021) Ni(OH)2-electrochemically reduced graphene oxide nanocomposites as anode electrocatalyst for direct ethanol fuel cell in alkaline media. Chem Phys Lett 763:138208. https://doi.org/10.1016/j.cplett.2020.138208
Moazzami N, Khadempir S, Karimi-Maleh H et al (2023) Enhanced methanol electrooxidation by electroactivated Pd/Ni(OH)2/N-rGO catalyst. Int J Hydrogen Energy 48:6680–6690. https://doi.org/10.1016/j.ijhydene.2022.01.248
Pop A, Manea F, Radovan C et al (2012) Non-enzymatic electrochemical detection of glycerol on boron-doped diamond electrode. Analyst 137:641–647. https://doi.org/10.1039/C2AN15645H
Mocaka J, Bonda AM, Mitchella S, Scollaryb G (1997) A statistical overview of standard (IUPAC and ACS) and new procedures for determining the limits of detection and quantification: application to voltammetric and stnp** techniques. Pure Appl Chern 69:297–328
Li N, Zhou Q, Li X et al (2014) Electrochemical detection of free glycerol in biodiesel using electrodes with single gold particles in highly ordered SiO2 cavities. Sens Actuators B Chem 196:314–320. https://doi.org/10.1016/j.snb.2014.02.017
Maruta AH, Paixão TRLCLC (2012) Flow injection analysis of free glycerol in biodiesel using a copper electrode as an amperometric detector. Fuel 91:187–191. https://doi.org/10.1016/j.fuel.2011.06.071
Agência Nacional do Petroléo GN e B (2014) Resolução ANP No 45 DE 25/08/2014. In: Dou. https://www.legisweb.com.br/legislacao/?id=274064. Accessed 1 Jul 2021
Thompson M, Ellison SLR, Wood R (2002) Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC technical report). Pure Appl Chem 74:835–855. https://doi.org/10.1351/pac200274050835
Acknowledgements
The authors would like to thank the Laboratory of Spectroscopy (Institute of Chemistry, UNESP, Brazil) for providing the SEM facilities to conduct the experiments.
Funding
This study was supported by the Fundação Amparo à Pesquisa do Estado de São Paulo (FAPESP) (Proc. 2017/17559-1 and Proc. 2017/09123-9).
Author information
Authors and Affiliations
Contributions
JPJO contributed to investigation, methodology, conceptualization, data curation, writing—original draft, and writing—review & editing. ACS contributed to formal analysis, data curation, writing—review & editing. MSPS: formal analysis, data curation, writing—review & editing. AFZ contributed to formal analysis, writing—review & editing. GBT contributed to formal analysis, writing—review & editing. RJS contributed to formal analysis, project administration, funding acquisition, and writing—review & editing. LLP contributed to conceptualization, methodology, formal analysis, writing—review & editing, supervision, project administration, and funding acquisition. All authors participated in the discussion, contributing to the final manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Oliveira, J.P.J., de Sá, A.C., de Sousa, M.S.P. et al. Glycerol determination by chronoamperometry using Ni(OH)2/RGO on carbon paste electrode. J Appl Electrochem 53, 2469–2482 (2023). https://doi.org/10.1007/s10800-023-01928-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-023-01928-6