Abstract
Purpose
To evaluate retinal and choroidal microvascular structures using optical coherence tomography angiography (OCTA) in patients with anemia and polycythemia vera (PV).
Methods
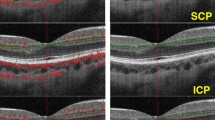
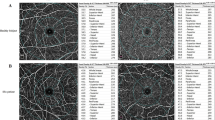
In this prospective study, 142 patients (142 eyes) were enrolled and divided into 3 groups: the anemia (n = 56), PV (n = 46), and healthy groups (n = 40, controls). For each patient, 6- × 6-mm macular angiography images were taken using an OCTA system (optovue, Inc., Fremont). For each eye analyzed, the software automatically measured vessel density (VD) in the superficial capillary plexus (SCP), deep capillary plexus (DCP; superior, nasal, temporal, and inferior quadrants), choriocapillaris (CC), and foveal avascular zone (FAZ).
Results
The OCTA analysis revealed that the VD of the DCP was significantly decreased in the superior and inferior hemispheres of the whole area, multiple quadrants of the perifovea, and CC with a 1-mm2 flow area in the anemia group compared with the PV group (p < 0.017), but the VD of the SCP did not show any significant difference in any quadrant between the two groups (p > 0.017). When compared with the healthy group, the anemia and PV groups showed a significant difference in multiple quadrants of the parafovea and temporal quadrant of the perifovea in the VD of the SCP and CC with a 2-mm2 flow area (p < 0.017). The FAZ and non-flow area did not manifest any significant difference between the groups (p > 0.017). The hemoglobin concentrations in the anemia, PV, and healthy groups were 8.11 ± 1.33, 17.5 ± 1.2, and 15.6 ± 0.73 g/dL, respectively, indicating statistically significant differences between the groups (p < 0.001).
Conclusion
In this study, quantitative OCTA analysis revealed a higher tendency for retinal and choroidal microvascular morphological changes in patients with anemia and PV. The outcomes of the current investigation can provide new insights into the retinal and choroidal pathophysiologies found in patients with hemoglobin abnormalities.




Similar content being viewed by others
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Morris CM, Candy JM, Oakley AE et al (1992) Histochemical distribution of non-haem iron in the human brain. Acta Anat (Basel) 144:235–257
He X, Hanh P, Iacovelli J et al (2007) Iron homeostasis and toxicity in retinal degeneration. Prog Retin Eye Res 26:649–673
Todorich B, Pasquini JM, Garcia CI et al (2009) Oligodendrocytes and myelination: the role of iron. Glia 57:467–478
DeMaman AS, Melo P, Homem JM et al (2010) Effectiveness of iron repletion in the diet for the optic nerve development of anaemic rats. Eye (Lond) 24:901–908
DeMaman AS, Homem JM, Lachat JJ (2008) Early iron deficiency produces persistent damage to visual tracts in Wistar rats. Nutr Neurosci 11:283–289
Hare D, Ayton S, Bush A, Lei P (2013) A delicate balance: iron metabolism and diseases of the brain. Front Aging Neurosci 5(34):1–19
Youdim MB (2008) Brain iron deficiency and excess; cognitive impairment and neurodegeneration with involvement of striatum and hippocampus. Neurotox Res 14:45–56
Schichi H (1969) Microsomal electron transport system of bovine retinal pigment epithelium. Exp Eye Res 8:60–68
Duzgun E, Demir N, Alkan AA et al (2022) Retinochoroidal vascular plexuses in patients with iron deficiency anaemia. Clin Exp Optom 105:326–332
Simsek A, Tekin M, Bilen A et al (2016) Evaluation of choroidal thickness in children with iron deficiency anemia. Investig Ophthalmol Vis Sci 57:5940–5944
Kacer B, Hattenbach LO, H¨orle S, et al (2001) Central retinal vein occlusion and nonarteritic ischemic optic neuropathy in 2 patients with mild iron deficiency anemia. Ophthalmologica 215:128–131
Carraro MC, Rossetti L, Gerli GC (2001) Prevalence of retinopathy in patients with anemia or thrombocytopenia. Eur J Haematol 67:238–244
Spivak JL (2019) How I treat polycythemia vera. Blood 134:341–352
Dameshek W (1951) Some speculations on the myeloproliferative syndromes [editorial]. Blood, pp 6372–5
Liisborg C, Hasselbalch HC, Sørensen TL (2020) Ocular manifestations in patients with philadelphia-negative myeloproliferative neoplasms. Cancers (Basel) 12:573
Barabas AP, Offen DN, Meinhard EA (1973) The arterial complications of polycythaemia vera. Br J Surg 60:183–187
Esmon CT (2009) Basic mechanisms and pathogenesis of venous thrombosis. Blood Rev 23:225e9
Jiaa Y, Bailey S, Hwang TS et al (2015) Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc Natl Acad Sci U S A 112:E2395–E2402
Alnawaiseh M, Eckardt F, Mihailovic N et al (2021) Ocular perfusion in patients with reduced left ventricular ejection fraction measured by optical coherence tomography angiography. Graefe’s Arch Clin Exp Ophthalmol 259:3605–3611
Tefferi A, Vannucchi AM, Barbui T (2021) Polycythemia vera: historical oversights, diagnostic details, and therapeutic views. Leukemia 35:3339–3351
Yang HS, Joe SG, Kim JG (2013) Delayed choroidal and retinal blood flow in polycythaemia vera patients with transient ocular blindness: a preliminary study with fluorescein angiography. Br J Haematol 161:745–747
Rao K, Shenoy SB, Kamath Y (2016) Central retinal artery occlusion as a presenting manifestation of polycythaemia vera. BMJ Case Rep 2016:3–5
Tan SS, Samsudin A, Thavaratnam LK (2022) Lights off, lights on: amaurosis fugax in polycythemia. Cureus 14:10–14
Regensburger J, Rauchegger R, Loacker L (2022) Intermittent retinal artery occlusions as the first clinical manifestation of polycythemia vera: a case report. BMC Ophthalmol 22:1–6
Menke MN, Feke GT, McMeel JW (2008) Effect of plasmapheresis on hyperviscosity-related retinopathy and retinal hemodynamics in patients with Waldenstrom’s macroglobulinemia. Invest Ophthalmol Vis Sci 49:1157–1160
Akdoğan M, Dogan M, Gobeka HH et al (2022) Optical coherence tomography angiographic assessment of polycythaemia vera-related retinal microvascular morphological changes: a cross-sectional case-control study. Turkiye Klin J Ophthalmol 31:152–157
Elstrott B, Khan L, Olson et al (2020) The role of iron repletion in adult iron deficiency anemia and other diseases. Eur J Haematol 104:153–161
Miller JL (2013) Iron deficiency anemia: a common and curable disease. Cold Spring Harb Perspect Med 3:a011866
Algarin C, Peirano P, Garrido M et al (2003) Iron deficiency anemia in infancy: long-lasting effects on auditory and visual system functioning. Pediatr Res 53:217–223
Korkmaz MF, Can ME, Kazancı EG (2020) Effects of iron deficiency anemia on peripapillary and macular vessel density determined using optical coherence tomography angiography on children. Graefe’s Arch Clin Exp Ophthalmol 258:2059–2068
Hwang TS, Jia Y, Gao SS et al (2015) Optical coherence tomography angiography features of diabetic retinopathy. Retina 35:367–373
Takase N, Nozaki M, Kato A et al (2015) Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina 35:2377–2383
de Carlo TE, Chin AT, Bonini Filho MA et al (2015) Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina 35:2364–2370
Adhi M, Filho MA, Louzada RN et al (2016) Retinal capillary network and foveal avascular zone in eyes with vein occlusion and fellow eyes analyzed with optical coherence tomography angiography. Invest Ophthalmol Vis Sci 57:486–494
Zhu J, Merkle CV, Bernucci MT et al (2017) Can OCT angiography be made a quantitative blood measurement tool? Appl Sci 7:687
Almeida OP, Flicker L (2001) The mind of a failing heart: a systematic review of the association between congestive heart failure and cognitive functioning. Intern Med J 31:290–295
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
SCD wrote the main manuscript SK, TF, and MYT reviewed the manuscript
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethical Committee of Ankara Etlik City Hospital.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Celik Dulger, S., Cevik Kaya, S., Fen, T. et al. Effects of hemoglobin concentration on retinochoroidal vascular plexuses: an optical coherence tomography angiography study. Int Ophthalmol 44, 117 (2024). https://doi.org/10.1007/s10792-024-03029-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10792-024-03029-5