Abstract
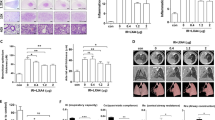
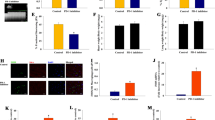
Cancer therapy has entered a new era with the use of programmed cell death protein 1 (PD-1) immune checkpoint inhibitors. When combined with thoracic radiotherapy, it demonstrates synergistic anti-tumor effects and potentially worsens radiation-induced myocardial fibrosis (RIMF). RIMF is the final stage of radiation-induced heart disease (RIHD) and a potentially fatal clinical complication of chest radiotherapy. It is characterized by decreased ventricular elasticity and distensibility, which can result in decreased ejection fraction, heart failure, and even sudden cardiac death. Pyroptosis, a type of programmed cell death, is mediated by members of the gasdermin (GSDM) family and has been associated with numerous cardiac disorders. The effect of pyroptosis on myocardial fibrosis caused by a combination of radiotherapy and PD-1 inhibitors remains uncertain. In this study, a 6MV X-ray of 20 Gy for local heart irradiation was used in the RIHD mouse model. We noticed that PD-1 inhibitors aggravated radiation-induced cardiac dysfunction and RIMF, concurrently enhancing the presence of CD8+ T lymphocytes in the cardiac tissue. Additionally, our findings indicated that the combination of PD-1 inhibitor and thoracic radiation can stimulate caspase-1 to cleave GSDMD, thereby regulating pyroptosis and liberating interleukin-8 (IL-18). In the myocardium of mice, the manifestation of pyroptosis mediated by GSDMD is accompanied by the buildup of proteins associated with fibrosis, such as collagen I, transforming growth factor β1 (TGF-β1), interleukin-6 (IL-6), vascular endothelial growth factor (VEGF), and tumor necrosis factor α (TNF-α). Moreover, it was discovered that TFG-β1 induced the phosphorylation of Smad2/Smad3 when the cardiac underwent PD-1 inhibitor in conjunction with thoracic irradiation (IR). The findings of this research indicate that PD-1 inhibitor worsen RIMF in mice by triggering GSDMD-induced pyroptosis and influencing the TGF-β1/Smads pathway. While using the caspase-1 inhibitor Z-YVAD-FMK, RIMF can be alleviated. Blocking GSDMD may be a viable strategy for managing myocardial fibrosis caused by the combination of PD-1 inhibitors and radiotherapy.







Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Chen, D.S., and I. Mellman. 2017. Elements of cancer immunity and the cancer-immune set point. Nature 541: 321–330. https://doi.org/10.1038/nature21349.
Reck, M., D. Rodriguez-Abreu, A.G. Robinson, et al. 2016. Pembrolizumab versus chemotherapy for pd-l1-positive non-small-cell lung cancer. New England Journal of Medicine 375: 1823–1833. https://doi.org/10.1056/NEJMoa1606774.
Motzer, R.J., B. Escudier, D.F. McDermott, et al. 2015. Nivolumab versus everolimus in advanced renal-cell carcinoma. New England Journal of Medicine 373: 1803–1813. https://doi.org/10.1056/NEJMoa1510665.
Tang, B., X. Yan, X. Sheng, et al. 2019. Safety and clinical activity with an anti-PD-1 antibody JS001 in advanced melanoma or urologic cancer patients. Journal of Hematology & Oncology 12: 7. https://doi.org/10.1186/s13045-018-0693-2.
Gettinger, S.N., J. Choi, N. Mani, et al. 2018. A dormant TIL phenotype defines non-small cell lung carcinomas sensitive to immune checkpoint blockers. Nature Communications 9: 3196. https://doi.org/10.1038/s41467-018-05032-8.
Janjigian, Y.Y., K. Shitara, M. Moehler, et al. 2021. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 398: 27–40. https://doi.org/10.1016/S0140-6736(21)00797-2.
Salous, T., N.A. Shukla, S.K. Althouse, et al. 2023. A phase 2 trial of chemotherapy plus pembrolizumab in patients with advanced non-small cell lung cancer previously treated with a PD-1 or PD-L1 inhibitor: Big Ten Cancer Research Consortium BTCRC-LUN15-029. Cancer 129: 264–271. https://doi.org/10.1002/cncr.34565.
Cheng, Y., L. Han, L. Wu, et al. 2022. Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer: The ASTRUM-005 Randomized Clinical Trial. JAMA 328: 1223–1232. https://doi.org/10.1001/jama.2022.16464.
Forde, P.M., J. Spicer, S. Lu, et al. 2022. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. New England Journal of Medicine 386: 1973–1985. https://doi.org/10.1056/NEJMoa2202170.
Reck, M., D. Rodriguez-Abreu, A.G. Robinson, et al. 2021. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score >/= 50. Journal of Clinical Oncology 39: 2339–2349. https://doi.org/10.1200/JCO.21.00174.
O’Byrne, K.J., K.H. Lee, S.W. Kim, et al. 2022. First-line nivolumab + ipilimumab in advanced NSCLC: checkMate 227 subpopulation analyses in Asian patients. ESMO Open 7: 100394. https://doi.org/10.1016/j.esmoop.2022.100394.
Welsh, J.W., J.V. Heymach, C. Guo, et al. 2020. Phase 1/2 trial of pembrolizumab and concurrent chemoradiation therapy for limited-stage SCLC. Journal of Thoracic Oncology 15: 1919–1927. https://doi.org/10.1016/j.jtho.2020.08.022.
Altorki, N.K., T.E. McGraw, A.C. Borczuk, et al. 2021. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: A single-centre, randomised phase 2 trial. The lancet Oncology 22: 824–835. https://doi.org/10.1016/S1470-2045(21)00149-2.
Spigel, D.R., C. Faivre-Finn, J.E. Gray, et al. 2022. Five-year survival outcomes from the pacific trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. Journal of Clinical Oncology 40: 1301–1311. https://doi.org/10.1200/JCO.21.01308.
Yi, M., X. Zheng, M. Niu, et al. 2022. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Molecular Cancer 21: 28. https://doi.org/10.1186/s12943-021-01489-2.
Madan, R., R. Benson, D.N. Sharma, P.K. Julka, and G.K. Rath. 2015. Radiation induced heart disease: Pathogenesis, management and review literature. Journal of the Egyptian National Cancer Institute 27: 187–193. https://doi.org/10.1016/j.jnci.2015.07.005.
Zhang, Z., Y. Zhang, S. **a, et al. 2020. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 579: 415–420. https://doi.org/10.1038/s41586-020-2071-9.
Zhou, Z.H., K. Wang. He, et al. 2020. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. https://doi.org/10.1126/science.aaz7548.
Wang, H., J. Wei, Q. Zheng, et al. 2019. Radiation-induced heart disease: a review of classification, mechanism and prevention. International Journal of Biological Sciences 15: 2128–2138. https://doi.org/10.7150/ijbs.35460.
Zhu, H., F.X. Galdos, D. Lee, et al. 2022. Identification of pathogenic immune cell subsets associated with checkpoint inhibitor-induced myocarditis. Circulation 146: 316–335. https://doi.org/10.1161/CIRCULATIONAHA.121.056730.
Liu, X., S. **a, Z. Zhang, H. Wu, and J. Lieberman. 2021. channelling inflammation: gasdermins in physiology and disease. Nature Reviews Drug Discovery 20: 384–405. https://doi.org/10.1038/s41573-021-00154-z.
Zhaolin, Z., L. Guohua, W. Shiyuan, and W. Zuo. 2019. Role of pyroptosis in cardiovascular disease. Cell Proliferation 52: e12563. https://doi.org/10.1111/cpr.12563.
Jiang, Y., Y. Yang, Y. Hu, et al. 2022. Gasdermin D restricts anti-tumor immunity during PD-L1 checkpoint blockade. Cell Reports 41: 111553. https://doi.org/10.1016/j.celrep.2022.111553.
Liu, X., Z. Zhang, J. Ruan, et al. 2016. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535: 153–158. https://doi.org/10.1038/nature18629.
Pan, X.C.Y., Y.Y. Cen. Liu, et al. 2019. Dual role of triptolide in interrupting the nlrp3 inflammasome pathway to attenuate cardiac fibrosis. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms20020360.
Artlett, C.M. 2012. The Role of the NLRP3 Inflammasome in Fibrosis. Open Rheumatology Journal 6: 80–86. https://doi.org/10.2174/1874312901206010080.
Peng, D., M. Fu, M. Wang, Y. Wei, and X. Wei. 2022. Targeting TGF-beta signal transduction for fibrosis and cancer therapy. Molecular Cancer 21: 104. https://doi.org/10.1186/s12943-022-01569-x.
Wang, B., H. Wang, M. Zhang, et al. 2020. Radiation-induced myocardial fibrosis: Mechanisms underlying its pathogenesis and therapeutic strategies. Journal of Cellular and Molecular Medicine 24: 7717–7729. https://doi.org/10.1111/jcmm.15479.
Ren, L.L., X.J. Li, T.T. Duan, et al. 2023. Transforming growth factor-beta signaling: from tissue fibrosis to therapeutic opportunities. Chemico-Biological Interactions 369: 110289. https://doi.org/10.1016/j.cbi.2022.110289.
Che, H., Y. Wang, H. Li, et al. 2020. Melatonin alleviates cardiac fibrosis via inhibiting lncRNA MALAT1/miR-141-mediated NLRP3 inflammasome and TGF-beta1/Smads signaling in diabetic cardiomyopathy. The FASEB Journal 34: 5282–5298. https://doi.org/10.1096/fj.201902692R.
Ge, C., Y. Zhao, Y. Liang, and Y. He. 2022. Silencing of TLR4 inhibits atrial fibrosis and susceptibility to atrial fibrillation via downregulation of NLRP3-TGF-beta in spontaneously hypertensive rats. Disease Markers 2022: 2466150. https://doi.org/10.1155/2022/2466150.
Zhang, H., J. Lin, Y. Shen, et al. 2022. Protective effect of crocin on immune checkpoint inhibitors-related myocarditis through inhibiting NLRP3 mediated pyroptosis in cardiomyocytes via NF-kappaB pathway. Journal of Inflammation Research 15: 1653–1666. https://doi.org/10.2147/JIR.S348464.
Zhang, Y., Z. Yao, Y. **ao, X. Zhang, and J. Liu. 2022. Downregulated XBP-1 rescues cerebral ischemia/reperfusion injury-induced pyroptosis via the NLRP3/caspase-1/GSDMD Axis. Mediators of Inflammation 2022: 8007078. https://doi.org/10.1155/2022/8007078.
Shen, J., J.M. Wu, G.M. Hu, et al. 2020. Membrane nanotubes facilitate the propagation of inflammatory injury in the heart upon overactivation of the beta-adrenergic receptor. Cell Death and Disease. https://doi.org/10.1038/s41419-020-03157-7.
Li, J., Z. An, J. Song, et al. 2021. Fine particulate matter-induced lung in flammation is mediated by pyroptosis in mice. Ecotoxicology and Environmental Safety 219: 112351. https://doi.org/10.1016/j.ecoenv.2021.112351.
Chakraborty, R., J. Chandra, S. Cui, et al. 2018. CD8(+) lineage dendritic cells determine adaptive immune responses to inflammasome activation upon sterile skin injury. Experimental Dermatology 27: 71–79. https://doi.org/10.1111/exd.13436.
Laird, B.J., D. McMillan, R.J.E. Skipworth, et al. 2021. The emerging role of interleukin 1beta (IL-1beta) in cancer cachexia. Inflammation 44: 1223–1228. https://doi.org/10.1007/s10753-021-01429-8.
Lopez-Castejon, G., and D. Brough. 2011. Understanding the mechanism of IL-1beta secretion. Cytokine & Growth Factor Reviews 22: 189–195. https://doi.org/10.1016/j.cytogfr.2011.10.001.
Kaplanov, I., Y. Carmi, R. Kornetsky, et al. 2019. Blocking IL-1beta reverses the immunosuppression in mouse breast cancer and synergizes with anti-PD-1 for tumor abrogation. Proceedings of the National Academy of Sciences USA 116: 1361–1369. https://doi.org/10.1073/pnas.1812266115.
Nguyen, P.M., and T.L. Putoczki. 2019. Could the inhibition of IL-17 or IL-18 be a potential therapeutic opportunity for gastric cancer? Cytokine 118: 8–18. https://doi.org/10.1016/j.cyto.2018.01.008.
Celada, L.J., J.A. Kropski, J.D. Herazo-Maya. et al. 2018. PD-1 up-regulation on CD4(+) T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-beta1 production. Science Translational Medicine 10. https://doi.org/10.1126/scitranslmed.aar8356.
Iwakura, Y., H. Ishigame, S. Saijo, S. Nakae. 2011. Functional specialization of interleukin-17 family members. Immunity 34: 149–162. https://doi.org/10.1016/j.immuni.2011.02.012.
Chen, K., and J.K. Kolls. 2017. Interluekin-17A (IL17A). Gene 614: 8–14. https://doi.org/10.1016/j.gene.2017.01.016.
de Streel, G., and S. Lucas. 2021. Targeting immunosuppression by TGF-beta1 for cancer immunotherapy. Biochemical Pharmacology 192: 114697.https://doi.org/10.1016/j.bcp.2021.114697.
Batlle, E., and J. Massague. 2019. Transforming growth factor-beta signaling in immunity and cancer. Immunity 50: 924–940. https://doi.org/10.1016/j.immuni.2019.03.024.
Apte, R.S., D.S. Chen, N. Ferrara. 2019. VEGF in signaling and disease: beyond discovery and development. Cell 176: 1248–1264. https://doi.org/10.1016/j.cell.2019.01.021.
Iyer, A.K. Ramesh, V. Castro, C.A., et al. 2015. Nitric oxide mediates bleomycin-induced angiogenesis and pulmonary fibrosis via regulation of VEGF. Journal of Cellular Biochemistry 116: 2484–2493. https://doi.org/10.1002/jcb.25192.
Zou, C., W. Li, Y. Pan. et al. 2017. 11beta-HSD1 inhibition ameliorates diabetes-induced cardiomyocyte hypertrophy and cardiac fibrosis through modulation of EGFR activity. Oncotarget 8: 96263–96275. https://doi.org/10.18632/oncotarget.22015.
Chaudhary, N.I., G.J. Roth, F. Hilberg, et al. 2007. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. European Respiratory Journal 29: 976–985. https://doi.org/10.1183/09031936.00152106.
Venkatadri, R., A.K. Iyer, V. Ramesh. et al. 2017. MnTBAP Inhibits bleomycin-induced pulmonary fibrosis by regulating VEGF and wnt signaling. Journal of Cellular Physiology 232: 506–516. https://doi.org/10.1002/jcp.25608.
Carmeliet, P. 2005. VEGF as a key mediator of angiogenesis in cancer. Oncology 69 (3): 4–10. https://doi.org/10.1159/000088478.
Itatani, Y., K. Kawada, T. Yamamoto, and Y. Sakai. 2018. Resistance to anti-angiogenic therapy in cancer-alterations to anti-VEGF pathway. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms19041232.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 81960548] and the Guizhou Provincial Natural Science Foundation, China [ZK (2022) key040].
Author information
Authors and Affiliations
Contributions
Bibo Wu: Designed and performed the experiments, Data Analysis, Writing manuscript; Shasha Zhao: Conducted partial animal experiments; Writing Original Draft; **g Zhang: Analyzed the data and interpreted the experimental results; Yao Liu and Jie Bai: Data Analysis and modify the article; Gang Wang: Data Curation; Yu Wang and Han Jiang: Helped with Modeling in Animals; Yinxiang Hu: Guide radiation physics techniques; Weiwei Ouyang and Bing Lu: Resources, Supervision; Shengfa Su (Corresponding Author): Conceived the project, Funding Acquisition, Planned and directed this research Project.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Limitations
There are some limitations to this study. First of all, in most animal models established by researchers to explore the mechanism of RIHD, the irradiation method is to receive the same dose of whole-heart irradiation, which does not involve in vivo tumors. However, in clinical practice, the radiation dose received by different areas in the heart of patients with thoracic tumors varies greatly. A single dose of 20 Gy to the heart is not directly related to exposure to radiation therapy. Secondly, only male mice were studied, and the effects on female mice may be different. Thirdly, each of the analyses contained a very small number of mice.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, B., Zhao, S., Zhang, J. et al. PD-1 Inhibitor Aggravate Irradiation-Induced Myocardial Fibrosis by Regulating TGF-β1/Smads Signaling Pathway via GSDMD-Mediated Pyroptosis. Inflammation (2024). https://doi.org/10.1007/s10753-024-02056-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10753-024-02056-9