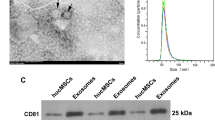
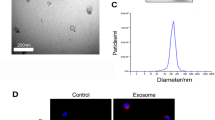
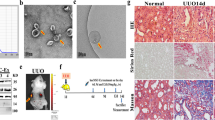
Abstract
Renal fibrosis, a progressive scarring of the kidney, lacks effective treatment. Human umbilical cord mesenchymal stem cell-derived exosomes (HucMSC-Exos) hold promise for treating kidney diseases due to their anti-inflammatory properties. This study investigates their potential to lessen renal fibrosis by targeting macrophage-to-myofibroblast transformation (MMT), a key driver of fibrosis. We employed a mouse model of unilateral ureteral obstruction (UUO) and cultured cells exposed to transforming growth factor-β (TGF-β) to mimic MMT. HucMSC-Exos were administered to UUO mice, and their effects on kidney function and fibrosis were assessed. Additionally, RNA sequencing and cellular analysis were performed to elucidate the mechanisms by which HucMSC-Exos inhibit MMT. HucMSC-Exos treatment significantly reduced kidney damage and fibrosis in UUO mice. They downregulated markers of fibrosis (Collagen I, vimentin, alpha-smooth muscle actin) and suppressed MMT (α-SMA + F4/80 + cells). Furthermore, ARNTL, a specific molecule, emerged as a potential target of HucMSC-Exos in hindering MMT and consequently preventing fibrosis. HucMSC-Exos effectively lessen renal fibrosis by suppressing MMT, suggesting a novel therapeutic strategy for managing kidney damage and fibrosis.







Similar content being viewed by others
Availability of Data and Materials
The datasets and all the relevant codes are available from the corresponding author.
Abbreviations
- HucMSC-Exos:
-
Human umbilical cord mesenchymal stem cell-derived exosomes
- MMT:
-
Macrophage-to-myofibroblast transformation
- UUO:
-
Unilateral ureteral obstruction
- TGF-β:
-
Transforming growth factor-β
- CKD:
-
Chronic Kidney Disease
- ESRD:
-
End stage renal disease
- MSC:
-
Mesenchymal Stem Cell
- HucMSCs:
-
Human umbilical cord mesenchymal stem cells
- TEM:
-
Transmission Electron Microscope
- NTA:
-
Nanoparticle Tracking Analysis
- FBS:
-
Fetal bovine serum
- FACS:
-
For fluorescence activated cell sorting
- FVS:
-
Fixable Viability Stain
- PMSF:
-
phenylmethylsulfonyl fluoride
- PVDF:
-
polyvinylidene difluoride
- ON:
-
Obstructive Nephropathy
- ARNTL:
-
Aryl Hydrocarbon Receptor Nuclear Translocator Like
References
Jager, K.J., C. Kovesdy, R. Langham, M. Rosenberg, V. Jha, and C. Zoccali. 2019. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney International 96 (5): 1048–1050. https://doi.org/10.1016/j.kint.2019.07.012.
GBD 2013 Mortality and Causes of Death Collaborators. 2015. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385 (9963): 117–171. https://doi.org/10.1016/S0140-6736(14)61682-2.
Huang, R., P. Fu, and L. Ma. 2023. Kidney fibrosis: from mechanisms to therapeutic medicines. Signal Transduction and Targeted Therapy 8 (1): 129. https://doi.org/10.1038/s41392-023-01379-7.
Huen, S.C., and L.G. Cantley. 2017. Macrophages in renal injury and repair. Annual Review of Physiology 79: 449–469. https://doi.org/10.1146/annurev-physiol-022516-034219.
Wang, Y., and D.C. Harris. 2011. Macrophages in renal disease. Journal of the American Society of Nephrology 22 (1): 21–27. https://doi.org/10.1681/ASN.2010030269.
Tang, P.M., D.J. Nikolic-Paterson, and H.Y. Lan. 2019. Macrophages: Versatile players in renal inflammation and fibrosis. Nature Reviews. Nephrology 15 (3): 144–158. https://doi.org/10.1038/s41581-019-0110-2.
Wen, Y., H.R. Yan, B. Wang, and B.C. Liu. 2021. Macrophage heterogeneity in kidney injury and fibrosis. Frontiers in Immunology 12: 681748. https://doi.org/10.3389/fimmu.2021.681748.
Falke, L.L., S. Gholizadeh, R. Goldschmeding, R.J. Kok, and T.Q. Nguyen. 2015. Diverse origins of the myofibroblast—implications for kidney fibrosis. Nature Reviews. Nephrology 11 (4): 233–244. https://doi.org/10.1038/nrneph.2014.246.
Meng, X.M., S. Wang, X.R. Huang, et al. 2016. Inflammatory macrophages can transdifferentiate into myofibroblasts during renal fibrosis. Cell Death and Disease 7 (12): e2495. https://doi.org/10.1038/cddis.2016.402.
Wang, Y.Y., H. Jiang, J. Pan, et al. 2017. Macrophage-to-myofibroblast transition contributes to interstitial fibrosis in chronic renal allograft injury. Journal of the American Society of Nephrology 28 (7): 2053–2067. https://doi.org/10.1681/ASN.2016050573.
Ouyang, X., X. Han, Z. Chen, J. Fang, X. Huang, and H. Wei. 2018. MSC-derived exosomes ameliorate erectile dysfunction by alleviation of corpus cavernosum smooth muscle apoptosis in a rat model of cavernous nerve injury. Stem Cell Research & Therapy 9 (1): 246. https://doi.org/10.1186/s13287-018-1003-1.
Yu, Y., M. Chen, Q. Guo, et al. 2023. Human umbilical cord mesenchymal stem cell exosome-derived miR-874–3p targeting RIPK1/PGAM5 attenuates kidney tubular epithelial cell damage. Cellular and Molecular Biology Letters 28 (1): 12. https://doi.org/10.1186/s11658-023-00425-0.
Song, Y., B. Wang, X. Zhu, et al. 2021. Human umbilical cord blood-derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biology and Toxicology 37 (1): 51–64. https://doi.org/10.1007/s10565-020-09530-8.
Costello, H.M., J.G. Johnston, A. Juffre, G.R. Crislip, and M.L. Gumz. 2022. Circadian clocks of the kidney: Function, mechanism, and regulation. Physiological Reviews 102 (4): 1669–1701. https://doi.org/10.1152/physrev.00045.2021.
Zha, M., T. Tian, W. Xu, et al. 2020. The circadian clock gene Bmal1 facilitates cisplatin-induced renal injury and hepatization. Cell Death and Disease 11 (6): 446. https://doi.org/10.1038/s41419-020-2655-1.
Zhang, J., C. Liu, Q. Liang, et al. 2021. Postnatal deletion of Bmal1 in mice protects against obstructive renal fibrosis via suppressing Gli2 transcription. The FASEB Journal 35 (5): e21530. https://doi.org/10.1096/fj.202002452R.
Liu, B., F. Ding, D. Hu, et al. 2018. Human umbilical cord mesenchymal stem cell conditioned medium attenuates renal fibrosis by reducing inflammation and epithelial-to-mesenchymal transition via the TLR4/NF-κB signaling pathway in vivo and in vitro [published correction appears in Stem Cell Res Ther. 2018 Mar 22;9(1):76]. Stem Cell Research and Therapy 9 (1): 7. https://doi.org/10.1186/s13287-017-0760-6.
Wang, F., C. Yang, J. Long, et al. 2019. Executive summary for the 2015 annual data report of the china kidney disease network (CK-NET) [published correction appears in kidney int. 2019 Aug; 96(2):525]. Kidney International 95 (3): 501–505. https://doi.org/10.1016/j.kint.2018.11.011.
de Cos, M., M. **pell, A. García-Herrera, et al. 2022. Assessing and counteracting fibrosis is a cornerstone of the treatment of CKD secondary to systemic and renal limited autoimmune disorders. Autoimmunity Reviews 21 (3): 103014. https://doi.org/10.1016/j.autrev.2021.103014.
Collins, A.J., R.N. Foley, D.T. Gilbertson, and S.C. Chen. 2009. The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clinical Journal of the American Society of Nephrology 4 (Suppl 1): S5–S11. https://doi.org/10.2215/CJN.05980809.
Moes, A.D., D.A. Hesselink, A.H. van den Meiracker, R. Zietse, and E.J. Hoorn. 2017. Chlorthalidone Versus Amlodipine for Hypertension in Kidney Transplant Recipients Treated With Tacrolimus: A Randomized Crossover Trial. American Journal of Kidney Diseases 69 (6): 796–804. https://doi.org/10.1053/j.ajkd.2016.12.017.
Qiu, Z., Z. Zhong, Y. Zhang, H. Tan, B. Deng, and G. Meng. 2022. Human umbilical cord mesenchymal stem cell-derived exosomal miR-335–5p attenuates the inflammation and tubular epithelial-myofibroblast transdifferentiation of renal tubular epithelial cells by reducing ADAM19 protein levels. Stem Cell Research & Therapy 13 (1): 373. https://doi.org/10.1186/s13287-022-03071-z.
Xu, S., Y.C. Cheuk, Y. Jia, et al. 2022. Bone marrow mesenchymal stem cell-derived exosomal miR-21a-5p alleviates renal fibrosis by attenuating glycolysis by targeting PFKM. Cell Death and Disease 13 (10): 876. https://doi.org/10.1038/s41419-022-05305-7.
Wei, J., Z. Xu, and X. Yan. 2022. The role of the macrophage-to-myofibroblast transition in renal fibrosis. Frontiers in Immunology 13: 934377. https://doi.org/10.3389/fimmu.2022.934377.
Ren, K. 2019. Exosomes in perspective: A potential surrogate for stem cell therapy. Odontology 107 (3): 271–284. https://doi.org/10.1007/s10266-018-0395-9.
**, C., Y. Cao, and Y. Li. 2023. Bone mesenchymal stem cells origin exosomes are effective against sepsis-induced acute kidney injury in rat model. International Journal of Nanomedicine 18: 7745–7758. https://doi.org/10.2147/IJN.S417627.
Eirin, A., and L.O. Lerman. 2021. Mesenchymal stem/stromal cell-derived extracellular vesicles for chronic kidney disease: are we there yet? Hypertension 78 (2): 261–269. https://doi.org/10.1161/HYPERTENSIONAHA.121.14596.
Zhu, Y.G., X.M. Feng, J. Abbott, et al. 2014. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 32 (1): 116–125. https://doi.org/10.1002/stem.1504.
Sun, G., G. Li, D. Li, et al. 2018. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Materials Science & Engineering, C: Materials for Biological Applications 89: 194–204. https://doi.org/10.1016/j.msec.2018.04.006.
Gibb, A.A., et al. 2020. Myofibroblasts and fibrosis: mitochondrial and metabolic control of cellular differentiation. Circulation Research 127 (3): 427–447. https://doi.org/10.1161/CIRCRESAHA.120.316958.
Falke, L.L., et al. 2015. Diverse origins of the myofibroblast—implications for kidney fibrosis. Nature reviews. Nephrology 11 (4): 233–44. https://doi.org/10.1038/nrneph.2014.246.
Schreibing, Felix, and Rafael Kramann. 2022. Map** the human kidney using single-cell genomics. Nature reviews. Nephrology 18 (6): 347–360. https://doi.org/10.1038/s41581-022-00553-4.
Shen, S., M. Zhang, X. Wang, et al. 2024. Single-cell RNA sequencing reveals S100a9hi macrophages promote the transition from acute inflammation to fibrotic remodeling after myocardial ischemia-reperfusion. Theranostics 14 (3): 1241–1259. https://doi.org/10.7150/thno.91180.
**a, S., Y. Huang, Y. Zhang, et al. 2023. Role of macrophage-to-myofibroblast transition in chronic liver injury and liver fibrosis. European Journal of Medical Research 28 (1): 502. https://doi.org/10.1186/s40001-023-01488-7.
Keller, M., J. Mazuch, U. Abraham, et al. 2009. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A. 106 (50): 21407–21412. https://doi.org/10.1073/pnas.0906361106.
Nguyen, K.D., S.J. Fentress, Y. Qiu, K. Yun, J.S. Cox, and A. Chawla. 2013. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 341 (6153): 1483–1488. https://doi.org/10.1126/science.1240636.
Silver, A.C., A. Arjona, M.E. Hughes, M.N. Nitabach, and E. Fikrig. 2012. Circadian expression of clock genes in mouse macrophages, dendritic cells, and B cells. Brain, Behavior, and Immunity 26 (3): 407–413. https://doi.org/10.1016/j.bbi.2011.10.001.
Shen, Y., L.R. Xu, D. Yan, et al. 2022. BMAL1 modulates smooth muscle cells phenotypic switch towards fibroblast-like cells and stabilizes atherosclerotic plaques by upregulating YAP1. Biochimica et Biophysica Acta, Molecular Basis of Disease 1868 (9): 166450. https://doi.org/10.1016/j.bbadis.2022.166450.
Hang, P.Z., J. Liu, J.P. Wang, et al. 2023. 7,8-Dihydroxyflavone alleviates cardiac fibrosis by restoring circadian signals via downregulating Bmal1/Akt pathway. European Journal of Pharmacology 938: 175420. https://doi.org/10.1016/j.ejphar.2022.175420.
Liang, Q., H. Xu, M. Liu, et al. 2022. Postnatal deletion of Bmal1 in cardiomyocyte promotes pressure overload induced cardiac remodeling in mice. Journal of the American Heart Association 11 (13): e025021. https://doi.org/10.1161/JAHA.121.025021.
Liu, C., S. Li, S. Ji, et al. 2023. Proximal tubular Bmal1 protects against chronic kidney injury and renal fibrosis by maintaining of cellular metabolic homeostasis. Biochimica et Biophysica Acta, Molecular Basis of Disease 1869 (1): 166572. https://doi.org/10.1016/j.bbadis.2022.166572.
Rey-Serra, C., J. Tituaña, T. Lin, et al. 2023. Reciprocal regulation between the molecular clock and kidney injury. Life Science Alliance 6 (10): e202201886. https://doi.org/10.26508/lsa.202201886.
Acknowledgements
The authors thank Chongqing Key Laboratory of Children Urogenital Department and Tissue Engineering and Department of Urology, Children’s Hospital of Chongqing Medical University.
Funding
This work was supported by Chongqing Science and Health Joint TCM Technology Innovation and Application Development Project (2020ZY023877), Program for Youth Innovation in Future Medicine, Chongqing Medical University (W0056), Basic Research and Frontier Exploration Project of Yuzhong District of Chongqing (20200126), the General Basic Research Project from the Ministry of Education Key Laboratory of Child Development and Disorders (GBRP-202109).
Author information
Authors and Affiliations
Contributions
DZ contributed to the conception and design; QG contributed to the investigation and writing of the original draft; PL, MC,YY and CR contributed to the collection and processing of the data; YW, ZZ, LS and XL provided technological guidance and revised the manuscript critically for important intellectual content; DH, YZ and GW conducted the statistical analysis; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All experimental procedures involving animals were conducted in accordance with the Basel Declaration and were approved by the ethics committee of Chongqing Medical University (approval no. CHCMU-IACUC20220429003, approval date 31 March 2022).
Consent to Participate
Not applicable
Consent for Publication
All authors approved the final manuscript and the submission to this journal.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, Q., Li, P., Chen, M. et al. Exosomes From Human Umbilical Cord Stem Cells Suppress Macrophage-to-myofibroblast Transition, Alleviating Renal Fibrosis. Inflammation (2024). https://doi.org/10.1007/s10753-024-02027-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10753-024-02027-0