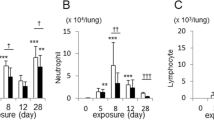
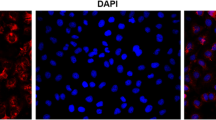
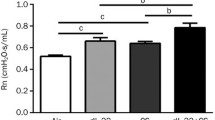
Abstract
Cigarette smoke (CS) facilitates adverse effects on the airway inflammation and treatment of asthma. Here, we investigated the mechanisms by which CS exacerbates asthma. The roles of IL-33 and IL-35 in asthma development were examined by treatment with IL-33 knockout (IL-33 KO) or transfection of adenovirus encoding IL-35 (Ad-IL-35) in a murine model of cigarette smoke-exposure asthma. Furthermore, the involvement of IL-33 and IL-35 in regulating DCs and Th2/Th17 cells was examined in a coculture system of DCs with CD4+ T cells. Additionally, we observed the effect of CpG-ODNs on the balance of IL-33 and IL-35. We show that CS and house dust mite (HDM) exposure induced IL-33 and suppressed IL-35 levels in cigarette smoke-exposure asthma in vivo and in vitro. Treatment with IL-33 KO or Ad-IL-35 significantly attenuated airway hyperreactivity, goblet hyperplasia, airway remodelling, and eosinophil and neutrophil infiltration in the lung tissues from asthmatic mice. Furthermore, we demonstrated reciprocal regulation between CS and HDM-modulated IL-33 and IL-35. Mechanistically, IL-33 KO (or anti-ST2) and Ad-IL-35 attenuated Th2- and Th17-associated inflammation by downregulating TSLP-DC signalling. Finally, administration of CpG-ODNs suppressed the expression of IL-33/ST2 and elevated the levels of IL-35, which is mainly derived from CD4+Foxp+ Tregs, to alleviate Th2- and Th17-associated inflammation by inhibiting the activation of BMDCs. Taken together, the IL-33/ST2 pathway drives the DC-Th2 and Th17 responses of cigarette smoke-exposure asthma, while IL-35 has the opposite effect. CpG-ODNs represent a potential therapeutic strategy for modulating the balance of IL-33 and IL-35 to suppress allergic airway inflammation.






Similar content being viewed by others
Availability of Data and Materials
The data used to support the findings of this study are included within the article and the supplementary information file.
References
Papi, A., C. Brightling, S.E. Pedersen, and H.K. Reddel. 2018. Asthma. Lancet 391: 783–800.
El-Husseini, Z.W., R. Gosens, F. Dekker, and G.H. Koppelman. 2020. The genetics of asthma and the promise of genomics-guided drug target discovery. The Lancet Respiratory Medicine 8: 1045–1056.
Wang, J., C. Janson, R. Jogi, B. Forsberg, T. Gislason, M. Holm, et al. 2021. A prospective study on the role of smoking, environmental tobacco smoke, indoor painting and living in old or new buildings on asthma, rhinitis and respiratory symptoms. Environmental Research 192: 110269.
Coogan, P.F., N. Castro-Webb, J. Yu, G.T. O’Connor, J.R. Palmer, and L. Rosenberg. 2015. Active and passive smoking and the incidence of asthma in the Black Women’s Health Study. American Journal of Respiratory and Critical Care Medicine 191: 168–176.
Lajunen, T.K., J.J. Jaakkola, and M.S. Jaakkola. 2013. The synergistic effect of heredity and exposure to second-hand smoke on adult-onset asthma. American Journal of Respiratory and Critical Care Medicine 188: 776–782.
Polosa, R., and N.C. Thomson. 2013. Smoking and asthma: Dangerous liaisons. European Respiratory Journal 41: 716–726.
Kiljander, T., T. Poussa, T. Helin, A. Jaakkola, K. Venho, and L. Lehtimäki. 2020. Symptom control among asthmatics with a clinically significant smoking history: A cross-sectional study in Finland. BMC Pulmonary Medicine 20: 88.
Polosa, R., C. Russo, P. Caponnetto, G. Bertino, M. Sarvà, T. Antic, et al. 2011. Greater severity of new onset asthma in allergic subjects who smoke: A 10-year longitudinal study. Respiratory Research 12: 16.
Dinarello, C.A. 2018. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunological Reviews 281: 8–27.
Savenije, O.E., John J. Mahachie, M., Granell R., Kerkhof M., Dijk F. N., de Jongste J. C., et al. 2014. Association of IL33-IL-1 receptor-like 1 (IL1RL1) pathway polymorphisms with wheezing phenotypes and asthma in childhood. The Journal of Allergy and Clinical Immunology 134: 170–177.
Cayrol, C., and J.P. Girard. 2018. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunological Reviews 281: 154–168.
Lee, J.H., K.L. Hailey, S.A. Vitorino, P.A. Jennings, T.D. Bigby, and E.C. Breen. 2019. Cigarette smoke triggers IL-33-associated inflammation in a model of late-stage chronic obstructive pulmonary disease. American Journal of Respiratory Cell and Molecular Biology 61: 567–574.
Hu, D. 2017. Role of anti-inflammatory cytokines IL-35 and IL-37 in asthma. Inflammation 40: 697–707.
Collison, L.W., C.J. Workman, T.T. Kuo, K. Boyd, Y. Wang, K.M. Vignali, et al. 2007. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450: 566–569.
Collison, L.W., V. Chaturvedi, A.L. Henderson, P.R. Giacomin, C. Guy, J. Bankoti, et al. 2010. IL-35-mediated induction of a potent regulatory T cell population. Nature Immunology 11: 1093–1101.
Teymouri, M., M. Pirro, F. Fallarino, M. Gargaro, and A. Sahebkar. 2018. IL-35, a hallmark of immune-regulation in cancer progression, chronic infections and inflammatory diseases. International Journal of Cancer 143: 2105–2115.
Wang, R.X., C.R. Yu, I.M. Dambuza, R.M. Mahdi, M.B. Dolinska, Y.V. Sergeev, et al. 2014. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nature Medicine 20: 633–641.
Liu, S., Y. Li, L. **a, H. Shen, and J. Lu. 2019. IL-35 prevent bone loss through promotion of bone formation and angiogenesis in rheumatoid arthritis. Clinical and Experimental Rheumatology 37: 820–825.
Peng, W., L. Wang, H. Zhang, Z. Zhang, and X. Chen. 2021. Effects of recombinant IL-35-BCG on Treg/Th17 cell imbalance and inflammatory response in asthmatic newborn mice induced by RSV. Inflammation 44: 2476–2485.
Chen, C., Y. Deng, H. Chen, X. Wu, S. Cheng, Y. Xu, et al. 2014. Decreased concentration of IL-35 in plasma of patients with asthma and COPD. Asian Pacific Journal of Allergy and Immunology 32: 211–217.
Jiang, S., F. Shan, Y. Zhang, L. Jiang, and Z. Cheng. 2018. Increased serum IL-17 and decreased serum IL-10 and IL-35 levels correlate with the progression of COPD. International Journal of Chronic Obstructive Pulmonary Disease 13: 2483–2494.
Hanagata, N. 2017. CpG oligodeoxynucleotide nanomedicines for the prophylaxis or treatment of cancers, infectious diseases, and allergies. International Journal of Nanomedicine 12: 515–531.
Sabatel, C., C. Radermecker, L. Fievez, G. Paulissen, S. Chakarov, C. Fernandes, et al. 2017. Exposure to bacterial CpG DNA protects from airway allergic inflammation by expanding regulatory lung interstitial macrophages. Immunity 46: 457–473.
Okajima, T., S. Shigemori, F. Namai, T. Ogita, T. Sato, and T. Shimosato. 2021. Free feeding of CpG-oligodeoxynucleotide particles prophylactically attenuates allergic airway inflammation and hyperresponsiveness in mice. Frontiers in Immunology 12: 738041.
Mohammadi-Shahrokhi, V., A. Rezaei, A. Andalib, A. Rahnama, A. Jafarzadeh, and N. Eskandari. 2017. Immunomodulatory effects of adjuvants CPG, MPLA, and BCG on the Derp2-induced acute asthma at early life in an animal model of BALB/c mice. Inflammation 40: 259–274.
Beeh, K.M., F. Kanniess, F. Wagner, C. Schilder, I. Naudts, A. Hammann-Haenni, et al. 2013. The novel TLR-9 agonist QbG10 shows clinical efficacy in persistent allergic asthma. The Journal of Allergy and Clinical Immunology 131: 866–874.
Krieg, A.M. 2006. Therapeutic potential of Toll-like receptor 9 activation. Nature Reviews Drug Discovery 5: 471–484.
Li, H.T., Z.G. Chen, Y.S. Lin, H. Liu, J. Ye, X.L. Zou, et al. 2018. CpG-ODNs and budesonide act synergistically to improve allergic responses in combined allergic rhinitis and asthma syndrome induced by chronic exposure to ovalbumin by modulating the TSLP-DC-OX40L axis. Inflammation 41: 1304–1320.
Li, H.T., Y.S. Lin, Q.M. Ye, X.N. Yang, X.L. Zou, H.L. Yang, et al. 2020. Airway inflammation and remodeling of cigarette smoking exposure ovalbumin-induced asthma is alleviated by CpG oligodeoxynucleotides via affecting dendritic cell-mediated Th17 polarization. International Immunopharmacology 82: 106361.
Ramaprakash, H., T. Shibata, K.E. Duffy, U.B. Ismailoglu, R.M. Bredernitz, A.P. Moreira, et al. 2011. Targeting ST2L potentiates CpG-mediated therapeutic effects in a chronic fungal asthma model. American Journal of Pathology 179: 104–115.
Thio, C.L., A.C. Lai, P.Y. Chi, G. Webster, and Y.J. Chang. 2019. Toll-like receptor 9-dependent interferon production prevents group 2 innate lymphoid cell-driven airway hyperreactivity. The Journal of Allergy and Clinical Immunology 144: 682-697.e689.
Tan, Y.Y., H.Q. Zhou, Y.J. Lin, L.T. Yi, Z.G. Chen, Q.D. Cao, et al. 2022. FGF2 is overexpressed in asthma and promotes airway inflammation through the FGFR/MAPK/NF-κB pathway in airway epithelial cells. Military Medical Research 9: 7.
Li, H.T., Q.M. Ye, Y.S. Lin, X.N. Yang, X.L. Zou, H.L. Yang, et al. 2021. CpG oligodeoxynucleotides attenuate RORγt-mediated Th17 response by restoring histone deacetylase-2 in cigarette smoke-exposure asthma. Cell & Bioscience 11: 92.
Zhang, H., S.L. Qiu, Q.Y. Tang, X. Zhou, J.Q. Zhang, Z.Y. He, et al. 2019. Erythromycin suppresses neutrophil extracellular traps in smoking-related chronic pulmonary inflammation. Cell Death & Disease 10: 678.
Myou, S., A.R. Leff, S. Myo, E. Boetticher, J. Tong, A.Y. Meliton, et al. 2003. Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase-TAT. Journal of Experimental Medicine 198: 1573–1582.
Le, A.V., J.Y. Cho, M. Miller, S. McElwain, K. Golgotiu, and D.H. Broide. 2007. Inhibition of allergen-induced airway remodeling in Smad 3-deficient mice. The Journal of Immunology 178: 7310–7316.
Lambrecht, B.N., and H. Hammad. 2017. The immunology of the allergy epidemic and the hygiene hypothesis. Nature Immunology 18: 1076–1083.
Han, Y., C. Yu, Y. Yu, and L. Bi. 2022. CD25+ B cells produced IL-35 and alleviated local inflammation during experimental periodontitis. Oral Diseases 28: 2248–2257.
Hammad, H., and B.N. Lambrecht. 2021. The basic immunology of asthma. Cell 184: 1469–1485.
Lanckacker, E.A., K.G. Tournoy, H. Hammad, G. Holtappels, B.N. Lambrecht, G.F. Joos, et al. 2013. Short cigarette smoke exposure facilitates sensitisation and asthma development in mice. European Respiratory Journal 41: 1189–1199.
Boonpiyathad, T., Z.C. Sözener, P. Satitsuksanoa, and C.A. Akdis. 2019. Immunologic mechanisms in asthma. Seminars in Immunology 46: 101333.
Dang, X., B. He, Q. Ning, Y. Liu, J. Guo, G. Niu, et al. 2020. Alantolactone suppresses inflammation, apoptosis and oxidative stress in cigarette smoke-induced human bronchial epithelial cells through activation of Nrf2/HO-1 and inhibition of the NF-κB pathways. Respiratory Research 21: 95.
Zi, Y., X. Wang, Y. Zi, H. Yu, Y. Lan, Y. Fan, et al. 2023. Cigarette smoke induces the ROS accumulation and iNOS activation through deactivation of Nrf-2/SIRT3 axis to mediate the human bronchial epithelium ferroptosis. Free Radical Biology & Medicine 200: 73–86.
Moffatt, M.F., I.G. Gut, F. Demenais, D.P. Strachan, E. Bouzigon, S. Heath, et al. 2010. A large-scale, consortium-based genomewide association study of asthma. New England Journal of Medicine 363: 1211–1221.
Wang, W., P. Li, Y.F. Chen, and J. Yang. 2015. A potential immunopathogenic role for reduced IL-35 expression in allergic asthma. Journal of Asthma 52: 763–771.
Huang, Q., C.D. Li, Y.R. Yang, X.F. Qin, J.J. Wang, X. Zhang, et al. 2021. Role of the IL-33/ST2 axis in cigarette smoke-induced airways remodelling in chronic obstructive pulmonary disease. Thorax thoraxjnl-2020-214712.
Kim, M.H., J.W. Kwon, J.H. Hahn, M. Kim, H.S. Chang, J.S. Park, et al. 2022. Circulating IL-32 and IL-33 levels in patients with asthma and COPD: A retrospective cross-sectional study. Journal of Thoracic Disease 14: 2437–2439.
Wechsler, M.E., M.K. Ruddy, I.D. Pavord, E. Israel, K.F. Rabe, L.B. Ford, et al. 2021. Efficacy and safety of itepekimab in patients with moderate-to-severe asthma. New England Journal of Medicine 385: 1656–1668.
Whitehead Gregory S., Rhonda H. Wilson, Keiko Nakano, Lauranell H. Burch, Hideki Nakano, Donald N. Cook. 2012. IL-35 production by inducible costimulator (ICOS)-positive regulatory T cells reverses established IL-17-dependent allergic airways disease. Journal of Allergy and Clinical Immunology 129: 207–215.e201–205.
Li, Y., X. Pan, X. Peng, S. Li, Y. Zhou, X. Zheng, et al. 2015. Adenovirus-mediated interleukin-35 gene transfer suppresses allergic airway inflammation in a murine model of asthma. Inflammation Research 64: 767–774.
Dong, J., C.K. Wong, Z. Cai, D. Jiao, M. Chu, and C.W. Lam. 2015. Amelioration of allergic airway inflammation in mice by regulatory IL-35 through dampening inflammatory dendritic cells. Allergy 70: 921–932.
Pan, X., K. Xu, Y. Li, X. Wang, X. Peng, M. Li, et al. 2019. Interleukin-35 expression protects against cigarette smoke-induced lung inflammation in mice. Biomedicine & Pharmacotherapy 110: 727–732.
Faustino, L.D., J.W. Griffith, R.A. Rahimi, K. Nepal, D.L. Hamilos, J.L. Cho, et al. 2020. Interleukin-33 activates regulatory T cells to suppress innate γδ T cell responses in the lung. Nature Immunology 21: 1371–1383.
Kohlgruber, A.C., S.T. Gal-Oz, N.M. LaMarche, M. Shimazaki, D. Duquette, H.F. Koay, et al. 2018. γδ T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nature Immunology 19: 464–474.
Guo, X.J., P. Dash, J.C. Crawford, E.K. Allen, A.E. Zamora, D.F. Boyd, et al. 2018. Lung γδ T cells mediate protective responses during neonatal influenza infection that are associated with type 2 immunity. Immunity 49: 531-544.e536.
Nie, M., Q. Zeng, L. **, Y. Tang, R. Luo, and W. Liu. 2021. The effect of IL-35 on the expression of nasal epithelial-derived proinflammatory cytokines. Mediators of Inflammation 2021: 1110671.
Merad, M., P. Sathe, J. Helft, J. Miller, and A. Mortha. 2013. The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annual Review of Immunology 31: 563–604.
Jaligama, S., V.S. Patel, P. Wang, A. Sallam, J. Harding, M. Kelley, et al. 2018. Radical containing combustion derived particulate matter enhance pulmonary Th17 inflammation via the aryl hydrocarbon receptor. Particle and Fibre Toxicology 15: 20.
Hammad, H., and B.N. Lambrecht. 2015. Barrier epithelial cells and the control of type 2 immunity. Immunity 43: 29–40.
Roan, F., K. Obata-Ninomiya, and S.F. Ziegler. 2019. Epithelial cell-derived cytokines: More than just signaling the alarm. The Journal of Clinical Investigation 129: 1441–1451.
Deckers, J., D. Sichien, M. Plantinga, J. Van Moorleghem, M. Vanheerswynghels, E. Hoste, et al. 2017. Epicutaneous sensitization to house dust mite allergen requires interferon regulatory factor 4-dependent dermal dendritic cells. The Journal of Allergy and Clinical Immunology 140: 1364-1377.e1362.
Agalioti, T., E.J. Villablanca, S. Huber, and N. Gagliani. 2018. T(H)17 cell plasticity: The role of dendritic cells and molecular mechanisms. Journal of Autoimmunity 87: 50–60.
Van Dyken, S.J., J.C. Nussbaum, J. Lee, A.B. Molofsky, H.E. Liang, J.L. Pollack, et al. 2016. A tissue checkpoint regulates type 2 immunity. Nature Immunology 17: 1381–1387.
Willart, M.A., K. Deswarte, P. Pouliot, H. Braun, R. Beyaert, B.N. Lambrecht, et al. 2012. Interleukin-1α controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. Journal of Experimental Medicine 209: 1505–1517.
Vocca, L., C. Di Sano, C.G. Uasuf, A. Sala, L. Riccobono, S. Gangemi, et al. 2015. IL-33/ST2 axis controls Th2/IL-31 and Th17 immune response in allergic airway diseases. Immunobiology 220: 954–963.
Huang, C.H., E.X. Loo, I.C. Kuo, G.H. Soh, D.L. Goh, B.W. Lee, et al. 2011. Airway inflammation and IgE production induced by dust mite allergen-specific memory/effector Th2 cell line can be effectively attenuated by IL-35. The Journal of Immunology 187: 462–471.
Vignali, D.A., and V.K. Kuchroo. 2012. IL-12 family cytokines: Immunological playmakers. Nature Immunology 13: 722–728.
Krieg, A.M., A.K. Yi, S. Matson, T.J. Waldschmidt, G.A. Bishop, R. Teasdale, et al. 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374: 546–549.
Kline, J.N., and Z.K. Ballas. 2002. DNA immunomodulation of asthma. Clinical Allergy and Immunology 16: 551–564.
Wirtz, S., C. Becker, M.C. Fantini, E.E. Nieuwenhuis, I. Tubbe, P.R. Galle, et al. 2005. EBV-induced gene 3 transcription is induced by TLR signaling in primary dendritic cells via NF-kappa B activation. The Journal of Immunology 174: 2814–2824.
Kim, D.H., J.H. Sohn, H.J. Park, J.H. Lee, J.W. Park, and J.M. Choi. 2016. CpG oligodeoxynucleotide inhibits cockroach-induced asthma via induction of IFN-γ+ Th1 cells or Foxp3+ regulatory T cells in the lung. Allergy, Asthma & Immunology Research 8: 264–275.
Senti, G., P. Johansen, Susanne Haug, C. Bull, C. Gottschaller, P. Müller et al. 2009. Use of A-type CpG oligodeoxynucleotides as an adjuvant in allergen-specific immunotherapy in humans: A phase I/IIa clinical trial. Clinical and Experimental Allergy 39: 562–570.
Acknowledgements
The authors express their gratitude to J Liu (Guangzhou Laide Liankang Biotechnology Co., Ltd.) for her assistance in establishing the mouse model. We sincerely thank Prof. Kefang Lai and Dr. Chuqin Huang (State Key Laboratory of Respiratory Disease, the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China) for invasive lung function assessment in mice. The current manuscript was revised by a native English speaking editor at American Journal Experts (AJE).
Funding
This study was funded by grants from National Natural Science Foundation of China (No. 81973984 and 81970017), and Basic and Applied Basic Research Foundation of Guangdong Province (No.2019A1515010918).
Author information
Authors and Affiliations
Contributions
J Liu, BT Su, and PZ Tao drafted the article. J Liu, BT Su, PZ Tao, XN Yang, L Zheng, YS Lin, XL Zou, HL Yang, and WB Wu performed the experiments and contributed to acquisition of data. J Liu, BT Su, PZ Tao, XN Yang, L Zheng, TT Zhang, and HT Li analysed the data. HT Li and TT Zhang designed the study and revised the article. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University.
Consent for Publication
The authors have consented to submission of the manuscript to Inflammation journal.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
**g Liu, Beiting Su, and Peizhi Tao have equal contribution to the article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, J., Su, B., Tao, P. et al. Interplay of IL-33 and IL-35 Modulates Th2/Th17 Responses in Cigarette Smoke Exposure HDM-Induced Asthma. Inflammation 47, 173–190 (2024). https://doi.org/10.1007/s10753-023-01902-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01902-6