Abstract
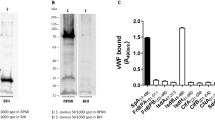
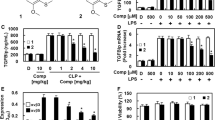
The aim of this study was to explore the effect of the anti-inflammatory protein TSG-6 induced by Staphylococcus bacteria on the regulation of chemokine function in endothelial cells by inhibiting the chemokine-glycosaminoglycan interaction. To cultivate human umbilical vein endothelial cells and Staphylococcus aureus bacteria, respectively, after the experiment is divided into the control group, S. aureus bacteria–induced group, S. aureus bacteria glycosaminoglycans about 1 mg/L sugar group, S. aureus bacteria glycosaminoglycans about 5 mg/L sugar group, and S. aureus bacteria glycosaminoglycans about 10 mg/L sugar group, E-selectin; intercellular adhesion molecule-1 (ICAM-1); monocyte chemoattractant protein-1 (MCP-1); interleukin-8 (IL-8) expression level; chemokine CXCL9, CXCL10, and CXCL11 mRNA and protein expression level; and TSG mRNA and protein expression level were determined in each cell; the endothelial cell proliferation and vascular endothelial cell function indicators were analyzed. The expression levels of E-selectin, ICAM-1, IL-8, MCP-1, and chemokines CXCL9, CXCL10, and CXCL11 mRNA and protein in each group at 6, 12, and 24 h were significantly different (P < 0.05). TSG mRNA and protein expression levels, endothelial cell proliferation ability, and vascular endothelial cell function were also significantly different (P < 0.05). The expression levels of E-selectin, ICAM-1, IL-8, MCP-1, endothelial cell proliferation ability, and vascular endothelial cell function in the Staphylococcus aureus–induced group were lower than those in the control group and the Staphylococcus aureus glycosaminoglycan group, and the mRNA and protein expression levels of chemokines CXCL9, CXCL10, and CXCL11, and TSG mRNA and protein expression levels were higher. With the increase of glycosaminoglycan concentration, the above indexes were improved. The anti-inflammatory protein TSG-6 induced by S. aureus can regulate the chemokine function of endothelial cells by inhibiting the chemokine-glycosaminoglycan interaction.



Similar content being viewed by others
Data Availability
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Day, A.J., and C.M. Milner. 2019. TSG-6: a multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol 78-79: 60–83.
Spinelli, F.M., D.L. Vitale, A. Icardi, I. Caon, A. Brandone, P. Giannoni, V. Saturno, A. Passi, M. García, I. Sevic, and L. Alaniz. 2019. Hyaluronan preconditioning of monocytes/macrophages affects their angiogenic behavior and regulation of TSG-6 expression in a tumor type-specific manner. FEBS J 286 (17): 3433–3449.
Jha, K.A., M. Pentecost, R. Lenin, J. Gentry, L. Klaic, N. Del Mar, A. Reiner, C.H. Yang, L.M. Pfeffer, N. Sohl, and R. Gangaraju. 2019. TSG-6 in conditioned media from adipose mesenchymal stem cells protects against visual deficits in mild traumatic brain injury model through neurovascular modulation. Stem Cell Res Ther 10 (1): 318.
Sammarco, G., M. Shalaby, S. Elangovan, L. Petti, G. Roda, S. Restelli, V. Arena, F. Ungaro, G. Fiorino, A.J. Day, S.D’. Alessio, and S. Vetrano. 2019. Hyaluronan accelerates intestinal mucosal healing through interaction with TSG-6. Cells 8 (9): 1074.
Li, X.Y., X.J. Weng, X.J. Li, X.Y, and Tian. 2020. TSG-6 inhibits the growth of keloid fibroblasts via mediating the TGF-β1/Smad signaling pathway. J Invest Surg. https://doi.org/10.1080/08941939.2020.1716894.
An, J.H., Q. Li, M.O. Ryu, A.R. Nam, D.H. Bhang, Y.C. Jung, W.J. Song, and H.Y. Youn. 2020. TSG-6 in extracellular vesicles from canine mesenchymal stem/stromal is a major factor in relieving DSS-induced colitis. PLoS One 15 (2): e0220756.
Idini, M., P. Wieringa, S. Rocchiccioli, G. Nieddu, N. Ucciferri, M. Formato, A. Lepedda, and L. Moroni. 2019. Glycosaminoglycan functionalization of electrospun scaffolds enhances Schwann cell activity. Acta Biomater 96: 188–202.
Yang, H., L. Wu, H. Deng, Y. Chen, H. Zhou, M. Liu, S. Wang, L. Zheng, L. Zhu, and X. Lv. 2020. Anti-inflammatory protein TSG-6 secreted by bone marrow mesenchymal stem cells attenuates neuropathic pain by inhibiting the TLR2/MyD88/NF-κB signaling pathway in spinal microglia. J Neuroinflammation 17 (1): 154.
Wang, Y., S. Yuan, J. Sun, Y. Gong, S. Liu, R. Guo, W. He, P. Kang, and R. Li. 2020. Inhibitory effect of the TSG-6 on the BMP-4/Smad signaling pathway and odonto/osteogenic differentiation of dental pulp stem cells. Biomed Pharmacother 128: 110266.
Miyaji, T., T. Takami, K. Fujisawa, T. Matsumoto, N. Yamamoto, and I. Sakaida. 2020. Bone marrow-derived humoral factors suppress oxidative phosphorylation, upregulate TSG-6, and improve therapeutic effects on liver injury of mesenchymal stem cells. J Clin Biochem Nutr 66 (3): 213–223.
Crijns, H., V. Vanheule, and P. Proost. 2020. Targeting chemokine-glycosaminoglycan interactions to inhibit inflammation. Front Immunol 11: 483.
Sandri, G., S. Rossi, M.C. Bonferoni, D. Miele, A. Faccendini, E. Del Favero, E. Di Cola, A. Cornaglia, C. Boselli, T. Luxbacher, L. Malavasi, L. Cantu, and F. Ferrari. 2019. Chitosan/glycosaminoglycan scaffolds for skin reparation. Carbohydr Polym 220: 219–227.
Chen, Y., H. Liu, Y. Wang, S. Yan, M. Yu, T. Jiang, and Z. Lv. 2019. Glycosaminoglycan from Apostichopus japonicus inhibits hepatic glucose production via activating Akt/FoxO1 and inhibiting PKA/CREB signaling pathways in insulin resistant hepatocytes. Food Funct 10 (11): 7565–7575.
Xu, T., J. Li, S. Zhang, Y. **, and R. Wang. 2019. Integration of diagnosis and treatment in the detection and kill of S. aureus in the whole blood. Biosens Bioelectron 142: 111507.
Wong Fok Lung, T., I.R. Monk, K.P. Acker, A. Mu, N. Wang, S.A. Riquelme, S. Pires, L.P. Noguera, F. Dach, S.J. Gabryszewski, B.P. Howden, and A. Prince. 2020. Staphylococcus aureus small colony variants impair host immunity by activating host cell glycolysis and inducing necroptosis. Nat Microbiol 5 (1): 141–153.
Feizabadi, N., J. Sarrafzadeh, M. Fathali, B. Vasaghi-Gharamaleki, M. Dadgoo, J. Kardan-Yamchi, H. Kazemian, S. Hesam-Shariati, and M.M. Feizabadi. 2019. The pulsed ultrasound strategy effectively decreases the S. aureus population of chronic rhinosinusitis patients. BMC Res Notes 12 (1): 576.
Bibek, G.C., G.S. Sahukhal, and M.O. Elasri. 2019. Role of the msaABCR operon in cell wall biosynthesis, autolysis, integrity, and antibiotic resistance in Staphylococcus aureus. Antimicrob Agents Chemother 63 (10): e00680–e00619.
Hayes, S.M., T.C. Biggs, S.P. Goldie, P.G. Harries, A.F. Walls, R.N. Allan, S.L.F. Pender, and R.J. Salib. 2020. Staphylococcus aureus internalization in mast cells in nasal polyps: characterization of interactions and potential mechanisms. J Allergy Clin Immunol 145 (1): 147–159.
Pang, D., S. Liao, W. Wang, L. Mu, E. Li, W. Shen, F. Liu, and Y. Zou. 2019. Destruction of the cell membrane and inhibition of cell phosphatidic acid biosynthesis in Staphylococcus aureus: an explanation for the antibacterial mechanism of morusin. Food Funct 10 (10): 6438–6446.
Author information
Authors and Affiliations
Contributions
ZY, LZ, HZ, and CHZ participated in writing the manuscript, designed the research study, analyzed the data, created images, and participated in the analysis. All authors have read and approved this manuscript for submission.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This research was authorized by the Ethics Committee of No. 2 Hospital of Baoding
Consent for Publication
Not applicable
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, Z., Zhang, L., Zhang, H. et al. The Anti-inflammatory Protein TSG-6 Induced by S. aureus Regulates the Chemokine Function of Endothelial Cells In Vitro by Inhibiting the Chemokine-Glycosaminoglycan Interaction. Inflammation 44, 1194–1202 (2021). https://doi.org/10.1007/s10753-021-01414-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01414-1