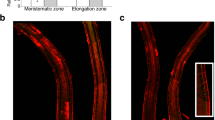
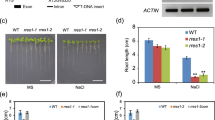
Abstract
Spatiotemporal regulation of reactive oxygen species (ROS) is fine-tuned at multiple levels, including transcriptional regulation. Arabidopsis copper amine oxidase ζ (CuAOζ) is localized in peroxisomes, and the CuAOζ-derived ROS is required for indole-3-butyric acid (IBA)-induced lateral root (LR) development. Here, we showed that both GATA2 and NIGT1.2 transcription factors interacted with the 5′ untranslated region (5′ UTR) of CuAOζ, using enhanced yeast one-hybrid analysis. NIGT1.2 was highly expressed in root tips and was upregulated by IBA. Phenotypic analysis, localization, and genetic studies demonstrate that NIGT1.2 positively regulated IBA-induced CuAOζ expression, ROS generation, and LR development. Furthermore, another transcription factor, UPBEAT1 (UPB1), regulated the basal expression of CuAOζ in roots, suggesting a putative functional regulation of CuAOζ in cellular proliferation and differentiation in the root. Together, our findings indicate that transcriptional regulation of peroxisomal CuAOζ plays an important role in determining CuAOζ spatiotemporal activity and ROS homeostasis within a develo** root.






Similar content being viewed by others
References
Adamowski M, Friml J (2015) PIN-dependent auxin transport: action, regulation, and evolution. Plant Cell 27:20–32
Alekhina OM, Vassilenko KS (2012) Translation initiation in eukaryotes: versatility of the scanning model. Biochemistry 77:1465–1477
An Z, **g W, Liu Y, Zhang W (2008) Hydrogen peroxide generated by copper amine oxidase is involved in abscisic acid-induced stomatal closure in Vicia faba. J Exp Bot 59:815–825
Angelini R, Cona A, Federico R, Fincato P, Tavladoraki P, Tisi A (2010) Plant amine oxidases ‘on the move’: an update. Plant Physiol Biochem 48:560–564
Bashandy T, Guilleminot J, Vernoux T, Caparros-Ruiz D, Ljung K, Meyer Y, Reichheld JP (2010) Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling. Plant Cell 22:376–391
Blomster T, Salojarvi J, Sipari N, Brosche M, Ahlfors R, Keinanen M, Overmyer K, Kangasjarvi J (2011) Apoplastic reactive oxygen species transiently decrease auxin signaling and cause stress-induced morphogenic response in Arabidopsis. Plant Physiol 157:1866–1883
Cerny M, Habanova H, Berka M, Luklova M, Brzobohaty B (2018) Hydrogen peroxide: its role in plant biology and crosstalk with signalling networks. Int J Mol Sci 19:2812
Chen YH, Chao YY, Hsu YY, Kao CH (2013) Heme oxygenase is involved in H2O2-induced lateral root formation in apocynintreated rice. Plant Cell Rep 32:219–226
Chen Z, Gu Q, Yu X, Huang L, Xu S, Wang R, Shen W, Shen W (2018) Hydrogen peroxide acts downstream of melatonin to induce lateral root formation. Ann Bot 121:1127–1136
Ditengou FA, Teale WD, Kochersperger P, Flittner KA, Kneuper I, van der Graaff E, Nziengui H, Pinosa F, Li X, Nitschke R (2008) Mechanical induction of lateral root initiation in Arabidopsis thaliana. Proc Natl Acad Sci USA 105:18818–18823
Du Y, Scheres B (2018) Lateral root formation and the multiple roles of auxin. J Exp Bot 69:155–167
Dubrovsky JG, Forde BG (2012) Quantitative analysis of lateral root development: pitfalls and how to avoid them. Plant Cell 24:4–14
Galvan-Ampudia CS, Testerink C (2011) Salt stress signals shape the plant root. Curr Opin Plant Biol 14:296–302
Giehl RF, Lima JE, von Wirén N (2012) Localized iron supply triggers lateral root elongation in Arabidopsis by altering the AUX1-mediated auxin distribution. Plant Cell 24:33–49
Hinnebusch AG, Ivanov IP, Sonenberg N (2016) Translational control by 5′-untranslated regions of eukaryotic mRNAs. Science 352:1413–1416
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Kazan K (2013) Auxin and the integration of environmental signals into plant root development. Ann Bot 112:1655–1665
Kiba T, Inaba J, Kudo T, Ueda N, Konishi M, Mitsuda N, Takiguchi Y, Kondou Y, Yoshizumi T, Ohme-Takagi M, Matsui M, Yano K, Yanagisawa S, Sakakibara H (2018) Repression of nitrogen starvation responses by members of the Arabidopsis GARP-type transcription factor NIGT1/HRS1 subfamily. Plant Cell 30:925–945
Krouk G, Lacombe B, Bielach A, Perrine-Walker F, Malinska K, Mounier E, Hoyerova K, Tillard P, Leon S, Ljung K, Zazimalova E, Benkova E, Nacry P, Gojon A (2010) Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev Cell 18:927–937
Lavenus J, Goh T, Roberts I, Guyomarc’h S, Lucas M, De Smet I, Fukaki H, Beeckman T, Bennett M, Laplaze L (2013) Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci 18:450–458
Li N, Sun L, Zhang L, Song Y, Hu P, Li C, Hao FS (2015) AtrbohD and AtrbohF negatively regulate lateral root development by changing the localized accumulation of superoxide in primary roots of Arabidopsis. Planta 241:591–602
Li BJ, Cai Q, Ma S, Li S, Zhang X, Yu Y (2018) Regulation of NPA and ACC on H2O2-induced Pea primary horizontal bending root. J Plant Growth Regul 37:246–254
Maeda Y, Konishi M, Kiba T, Sakuraba Y, Sawaki N, Kurai T, Ueda Y, Sakakibara H, Yanagisawa S (2018) A NIGT1-centred transcriptional cascade regulates nitrate signalling and incorporates phosphorus starvation signals in Arabidopsis. Nat Commun 9(1):1376
Manzano C, Pallero-Baena M, Casimiro I, De Rybel B, Orman-Ligeza B, Van Isterdael G, Beeckman T, Draye X, Casero P, Del Pozo JC (2014) The emerging role of reactive oxygen species signaling during lateral root development. Plant Physiol 165:1105–1119
Orosa-Puente B, Leftley N, von Wangenheim D, Banda J, Srivastava AK, Hill K, Truskina J, Bhosale R, Morris E, Srivastava M, Kümpers B, Goh T, Fukaki H, Vermeer JEM, Vernoux T, Dinneny JR, French AP, Bishopp A, Sadanandom A, Bennett MJ (2018) Root branching toward water involves posttranslational modification of transcription factor ARF7. Science 362:1407–1410
Overvoord P, Fukaki H, Beeckman T (2010) Auxin control of root development. Cold Spring Harbor Perspect Biol 2:a001537
Peret B, Larrieu A, Bennett MJ (2009) Lateral root emergence: a difficult birth. J Exp Bot 60:3637–3643
Pestova TV, Kolupaeva VG (2002) The roles of individual eukaryotic translation initiation factors in ribosomal scanning and initiation codon selection. Gene Dev 16:2906–2922
Petricka JJ, Winter CM, Benfey PN (2012) Control of Arabidopsis root development. Ann Rev Plant Biol 63:563–590
Planas-Portell J, Gallart M, Tiburcio AF, Altabella T (2013) Coppercontaining amine oxidases contribute to terminal polyamine oxidation in peroxisomes and apoplast of Arabidopsis thaliana. BMC Plant Biol 13:109
Qu Y, An Z, Zhuang B, **g W, Zhang Q, Zhang W (2014) Copper amine oxidase and phospholipase D act independently in abscisic acid (ABA)-induced stomatal closure in Vicia faba and Arabidopsis. J Plant Res 127:533–544
Qu Y, Wang Q, Guo J, Wang P, Song P, Jia Q, Zhang X, Kudla J, Zhang W, Zhang Q (2017) Peroxisomal CuAOζ and its product H2O2 regulate the distribution of auxin and IBA-dependent lateral root development in Arabidopsis. J Exp Bot 68:4851–4867
Reyes JC, Muro-Pastor MI, Florencio FJ (2004) The GATA family of transcription factors in Arabidopsis and rice. Plant Physiol 134:1718–1732
Su GX, Zhang WH, Liu YL (2006) Involvement of hydrogen peroxide generated by polyamine oxidative degradation in the development of lateral roots in soybean. J Integr Plant Biol 48:426–432
Tian Y, Fan M, Qin Z, Lv H, Wang M, Zhang Z, Zhou W, Zhao N, Li X, Han C (2018) Hydrogen peroxide positively regulates brassinosteroid signaling through oxidation of the BRASSINAZOLE-RESISTANT1 transcription factor. Nat Commun 9:1063
Tognetti VB, Muhlenbock P, Van Breusegem F (2012) Stress homeostasis-the redox and auxin perspective. Plant Cell Environ 35:321–333
Tsukagoshi H, Busch W, Benfey PN (2010) Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143:606–616
Wang P, Du Y, Li Y, Ren D, Song CP (2010) Hydrogen peroxide-mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cell 22:2981–2998
Wu C, Feng J, Wang R, Liu H, Yang H, Rodriguez PL, Qin H, Liu X, Wang D (2012) HRS1 acts as a negative regulator of abscisic acid signaling to promote timely germination of Arabidopsis seeds. PLoS ONE 7:e35764
Xuan W, Audenaert D, Parizot B, Moller BK, Njo MF, De Rybel B, De Rop G, Van Isterdael G, Mahonen AP, Vanneste S, Beeckman T (2015) Root cap-derived auxin pre-patterns the longitudinal axis of the Arabidopsis root. Curr Biol 25:1381–1388
Acknowledgements
We thank Philip N. Benfey (Duke University) for kindly providing upb1 and P35S:UPB1-3YFP seeds. The work was supported by grants from the Natural Science Foundation of Jiangsu Province (BK20160720) and the National Natural Science Foundation of China (31700237) and the Fundamental Research Funds for the Central Universities (Y0201700648) to Y.Q. and the National Natural Science Foundation of China (31970300) and the Fundamental Research Funds for the Central Universities (KYZ201858) to Q.Z.
Author information
Authors and Affiliations
Contributions
YQ and QZ designed the research; XL, XZ, YT, YH, SC, LX, and QZ performed research; and YQ and QZ analyzed data and wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qu, Y., Liu, X., Zhang, X. et al. Transcriptional regulation of Arabidopsis copper amine oxidase ζ (CuAOζ) in indole-3-butyric acid-induced lateral root development. Plant Growth Regul 89, 287–297 (2019). https://doi.org/10.1007/s10725-019-00535-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-019-00535-w