Abstract
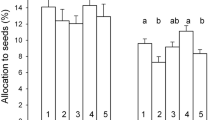
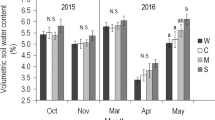
Most studies on consequences of environmental change focus on evolutionary and phenotypic plastic responses, but parental effects represent an additional mechanism by which organisms respond to their local environment. Parental effects can be adaptive if they enhance offsprings ability to cope with environments experienced by their parents, but can also be non-adaptive for instance when offspring from benign environments are just better provisioned and hence perform better than offspring from less benign environments. Parental effects originate from both the abiotic and biotic environmental variation. However, the effects of the parental abiotic and biotic environment are rarely studied together. We make use of an experimental set-up containing plots in a natural heath land, where summer precipitation was manipulated to reflect either ambient or drought conditions. In both plot types, competition from grasses was prevalent. We assessed survival and reproduction of Hieracium umbellatum offspring originating from ambient and drought plots grown in a factorial design with two levels of moisture (control and drought) and two levels of competition (grown with and without a local perennial grass). The maternal environment strongly affected offspring performance. Biomass and reproduction was higher in offspring from ambient plots in agreement with the hypothesis of a better maternal provisioning in the most benign environment. However, adding competition revealed potentially adaptive responses to survival, and altered allocation to reproduction in offspring from maternal drought plots. Under combined competition and drought (mimicking maternal drought plots), survival was only reduced in offspring from ambient plots, and offspring from drought plots survived best. When grown in competition under control watering conditions mimicking maternal ambient plots, offspring from drought plots (growing in an environment different from their maternal one) showed a 25% reduction in reproduction. Potential adaptive responses to the home maternal environment were only revealed when jointly manipulating levels of competition and water availability.




Similar content being viewed by others
References
Agrawal AA (2001) Transgenerational consequences of plant responses to herbivory: an adaptive maternal effect? Am Nat 157:555–569
Auge GA, Leverett LD, Edwards BR, Donohue K (2017) Adjusting phenotypes via within- and across-generational plasticity. New Phytol 216:343–349
Bataillon T, Galtier N, Bernard A et al (2016) A replicated climate change field experiment reveals rapid evolutionary response in an ecologically important soil invertebrate. Glob Chang Biol 22:2370–2379
Bates D, Mächler M, Bolker B et al (2015) Fitting linear mixed-effects models using lme4. J Stat Softw. https://doi.org/10.18637/jss.v067.i01
Beier C, Emmett B, Gundersen P et al (2004) Novel approaches to study climate change effects on terrestrial ecosystems at the field scale: drought and passive night time warming. Ecosystems 7:583–597
Bossdorf O, Richards CL, Pigliucci M (2008) Epigenetics for ecologists. Ecol Lett 11:106–115
Bulmer MG (1994) Theoretical evolutionary ecology. Sinauer Associates, Sunderland
Callaway RM (2007) Positive interactions and interdependence in plant communities. Springer, Dordrecht
Carroll SP, Hendry AP, Reznick DN et al (2007) Evolution on ecological time-scales. Funct Ecol 21:387–393
Cheptou P-O, Carrue O, Rouifed S, Cantarel A (2008) Rapid evolution of seed dispersal in an urban environment in the weed Crepis sancta. Proc Natl Acad Sci USA 105:3796–3799
Damgaard C, Riis-Nielsen T, Schmidt IK (2009) Estimating plant competition coefficients and predicting community dynamics from non-destructive pin-point data: a case study with Calluna vulgaris and Deschampsia flexuosa. Plant Ecol 201:687–697
de Jong T, Klinkhammer P (2005) Evolutionary ecology of plant reproductive strategies. Cambridge University Press, Cambridge
Franks SJ, Sim S, Weis AE (2007) Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc Natl Acad Sci USA 104:1278–1282
Galloway LF (2005) Maternal effects provide phenotypic adaptation to local environmental conditions. New Phytol 166:93–100
Galloway LF, Etterson JR (2007) Transgenerational plasticity is adaptive in the wild. Science 318:1134–1136
Hansen CF, Garcia MB, Ehlers BK (2013) Water availability and population origin affect the expression of the tradeoff between reproduction and growth in Plantago coronopus. J Evol Biol 26:993–1002
Herman JJ, Sultan SE (2011) Adaptive transgenerational plasticity in plants: case studies, mechanisms, and implications for natural populations. Front Plant Sci 2:102. https://doi.org/10.3389/fpls.2011.00102
Herman JJ, Sultan SE (2016) DNA methylation mediates genetic variation for adaptive transgenerational plasticity. Proc R Soc B 283:20160988
Herman JJ, Sultan SE, Horgan-Kobelski T et al (2012) Adaptive transgenerational plasticity in an annual plant: grandparental and parental drought stress enhance performance of seedlings in dry soil. Integr Comp Biol. https://doi.org/10.1093/icb/ics041
Holmstrup M, Sørensen JG, Schmidt IK et al (2013) Soil microarthropods are only weakly impacted after 13 years of repeated drought treatment in wet and dry heathland soils. Soil Biol Biochem 66:110–118
Huey RB, Berrigan D, Gilchrist GW et al (1999) Testing the adaptive significance of acclimation: a strong inference approach. Am Zool 39:323–336
Marshall DJ, Uller T (2007) When is a maternal effect adaptive? Oikos 116:1957–1963
Metz J, von Oppen J, Tielbörger K (2015) Parental environmental effects due to contrasting watering adapt competitive ability, but not drought tolerance, in offspring of a semi-arid annual Brassicaceae. J Ecol 103:990–997
Michalet R, Pugnaire FI (2016) Facilitation in communities: underlying mechanisms, community and ecosystem implications. Funct Ecol 30:3–9
Monaghan P (2008) Early growth conditions, phenotypic development and environmental change. Philos Trans R Soc Lond B Biol Sci 12:1635–1645
Mousseau TA, Fox CW (1998) Maternal effects as adaptations. Oxford University Press, Oxford
Nicotra AB, Atkin OK, Bonser SP et al (2010) Plant phenotypic plasticity in a changing climate. Trends Plant Sci 15:684–692
Nosalewicz A, Siecinska J, Smiech M et al (2016) Transgenerational effects of temporal drought stress on spring barley morphology and functioning. Environ Exp Bot 131:120–127
Penuelas J, Prieto P, Beier C et al (2007) Response of plant species richness and primary productivity in shrublands along a north-south gradient in Europe to seven years of experimental warming and drought: reduction in primary productivity in the heat and drought year of 2003. Glob Chang Biol 13:2563–2581
Roach DA, Wulff RD (1987) Maternal effects in plants. Annu Rev Ecol Syst 18:209–235
Rottstock T, Kummer V, Fischer M et al (2017) Rapid transgenerational effects in Knautia arvensis in response to plant community diversity. J Ecol 105:714–725
Shaw RG, Etterson JR (2012) Rapid climate change and the rate of adaptation: insight from experimental quantitative genetics. New Phytol 195:752–765
Sowerby A, Emmett BA, Tietema A et al (2008) Contrasting effects of repeated summer drought on soil carbon efflux in hydric and mesic heathland soils. Glob Change Biol 14:2388–2404
Stearns S (1992) The evolution of life histories. Oxford University Press, Oxford
Sultan SE, Barton K, Wilczek AM (2009) Contrasting patterns of transgenerational plasticity in ecologically distinct congeners. Ecology 90:1831–1839
terHorst CP, Lau JA (2011) Direct and indirect transgenerational effects alter plant-herbivore interactions. Evol Ecol. https://doi.org/10.1007/s10682-012-9560-8
Violle C, Castro H, Richarte J et al (2009) Intraspecific seed trait variations and competition: passive or adaptive response? Funct Ecol 23:612–620
Walther J, Harter DEV, Beierkuhnlein C et al (2016) Transgenerational effects of extreme weather: perennial plant offspring show modified germination, growth and stoichiometry. J Ecol 104:1032–1040
Acknowledgements
This work was financially supported by a grant from the Villum Foundation (PI BKE). We are grateful to L. Lauridsen, J. Rytter and technical staff at Bioscience Silkeborg for their help in the field and greenhouse, to C. Damgaard for statistical advice, and to S. Tomiolo and four anonymous reviewers for comments on previous versions of the manuscript. The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ehlers, B.K., Holmstrup, M., Schmidt, I.K. et al. Joint impact of competition, summer precipitation, and maternal effects on survival and reproduction in the perennial Hieracium umbellatum. Evol Ecol 32, 529–545 (2018). https://doi.org/10.1007/s10682-018-9953-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-018-9953-4