Abstract
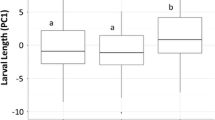
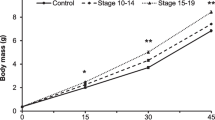
The timing of many life history events shows phenotypic plasticity in response to the risk of predation. Theory predicts that increased risk of mortality in an early stage should select for switching earlier, while a higher risk after the transition should select for switching later. Here we examined the effects of stage-specific predation risk on the timing of hatching of Rana temporaria. Embryos were exposed to chemical cues from either an egg predator (Haemopis sanguisuda) or a tadpole predator (Aeshna cyanea) to evaluate three specific hypotheses: (1) a fixed intermediate response, (2) a ‘fixed predator’ response (i.e., either anticipation or delay), and (3) a specific predator response (both anticipation and delay). Rana temporaria embryos did not discern between pre- and post-hatching specific predators, and they hatched prematurely regardless predator type. These results suggest that R. temporaria embryos respond to predation risk in a fixed way by hatching early, and that they use cues stemming from injured conspecifics, which provides a simple, conservative mechanism of risk assessment. In conclusion, our data are not anticipated by the theoretical consideration that organisms should spend less time in more dangerous environments, but they confirm an invariable adjustment of hatching time in response to an inscrutable predation risk (response to a fixed-predator) in connection with a consistent mechanism mediating the perception of predation risk.

Similar content being viewed by others
References
Alford RA (1999) Tadpoles: the biology of anuran larvae. In: McDiarmid RW, Altig R (eds) Ecology: resource use, competition, and predation. University of Chicago Press, Chicago, pp 240–278
Alford RA, Harris RN (1988) Effects of larval growth history on anuran metamorphosis. Am Nat 131:91–106. doi:10.1086/284775
Altwegg A, Reyer HU (2003) Fitness consequences of larval growth environment and timing of metamorphosis in waterfrogs. Evol Int J Org Evol 57:872–882
Álvarez D, Nicieza AG (2002) Effects of induced variation in anuran larval development on postmetamorphic energy reserves and locomotion. Oecologia 131:186–195. doi:10.1007/s00442-002-0876-x
Álvarez D, Nicieza AG (2006) Factors determining tadpole vulnerability to predators: can prior experience compensate for a suboptimal shape? Evol Ecol 20:523–534. doi:10.1007/s10682-006-9114-z
Benard MF (2004) Predator-induced phenotypic plasticity in organisms with complex life histories. Annu Rev Ecol Syst 35:651–673. doi:10.1146/annurev.ecolsys.35.021004.112426
Burnham KP, Anderson DR (2002) Model selection and inference: a practical information—theoretic approach. Springer, New York
Capellán E, Nicieza AG (2007a) Trade-offs across life stages: does predator-induced hatching plasticity reduce anuran post-metamorphic performance? Evol Ecol 21:445–458. doi:10.1007/s10682-006-9133-9
Capellán E, Nicieza AG (2007b) Non-equivalence of growth arrest induced by predation risk or food limitation: context-dependent compensatory growth in anuran tadpoles. J Anim Ecol 76:1026–1035. doi:10.1111/j.1365-2656.2007.01281.x
Chivers DP, Kiesecker JM, Marco A, DeVito J, Anderson MT, Blaustein AR (2001) Predator-induced life history changes in amphibians: egg predation induces hatching. Oikos 92:135–142. doi:10.1034/j.1600-0706.2001.920116.x
Gomez-Mestre I, Wiens JJ, Warkentin KM (2008) Evolution of adaptive plasticity: risk-sensitive hatching in neotropical leaf-breeding treefrogs. Ecol Monogr 78:205–224. doi:10.1890/07-0529.1
Gunzburger MS, Travis J (2005) Critical literature review of the evidence for unpalatability of amphibian eggs and larvae. J Herpetol 39:547–571. doi:10.1670/1-05A.1
Henrikson BI (1990) Predation on amphibian eggs and tadpoles by common predators in acidified lakes. Holarct Ecol 13:201–206
Ireland DH, Wirsing AJ, Murray DL (2007) Phenotypically plastic responses of green frog embryos to conflicting predation risk. Oecologia 152:162–168. doi:10.1007/s00442-006-0637-3
Johnson JB, Saenz D, Adams CK, Conner RN (2003) The influence of predator threat on the timing of a life-history switch point: predator-induced hatching in the southern leopard frog (Rana sphenocephala). Can J Zool 81:1608–1613. doi:10.1139/z03-148
Langerhans RB, DeWitt TJ (2002) Plasticity constrained: over-generalized induction cues cause maladaptive phenotypes. Evol Ecol Res 4:857–870
Laurila A, Pakkasmaa S, Crochet PA, Merila J (2002) Predator-induced plasticity in early life history and morphology in two anuran amphibians. Oecologia 132:524–530. doi:10.1007/s00442-002-0984-7
Li D (2002) Hatching responses of subsocial spitting spiders to predation risk. Proc R Soc Lond B Biol Sci 269:2155–2161. doi:10.1098/rspb.2002.2140
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS system for mixed models. SAS Institute Inc., Cary
Matsuda H, Hori M, Abrams P (1994) Effects of predator-specific defense on predator persistence and community complexity. Evol Ecol 8:628–638. doi:10.1007/BF01237846
Matsuda H, Hori M, Abrams P (1996) Effects of predator-specific defense on biodiversity and community complexity in two-trophic-level communities. Evol Ecol 10:13–28. doi:10.1007/BF01239343
Nicieza AG (2000) Interacting effects of predation risk and food availability on larval anuran behaviour and development. Oecologia 123:497–505. doi:10.1007/s004420000343
Nicieza AG, Metcalfe NB (1997) Growth compensation in juvenile Atlantic salmon: responses to depressed temperature and food availability. Ecology 78:2385–2400
Nicieza AG, Álvarez D, Atienza EMS (2006) Delayed effects of larval predation risk and food quality on anuran juvenile performance. J Evol Biol 19:1092–1103. doi:10.1111/j.1420-9101.2006.01100.x
Pinhero JC, Bates DM (2000) Mixed effects models in S and S-plus. Springer, New York
Relyea RA (2007) Getting out alive: how predators affect the decision to metamorphose? Oecologia 152:389–400. doi:10.1007/s00442-007-0675-5
Sih A, Moore RD (1993) Delayed hatching of salamander eggs in response to enhanced larval predation risk. Am Nat 142:947–960. doi:10.1086/285583
Stearns SC, Koella JC (1986) The evolution of phenotypic plasticity in life-history traits: predictions of reaction norms for age and size at maturity. Evolution Int J Org Evolution 40:893–913. doi:10.2307/2408752
Touchon JC, Gomez-Mestre I, Warkentin KM (2006) Hatching plasticity in two temperate anurans: responses to a pathogen and predation cues. Can J Zool 84:556–563. doi:10.1139/Z06-058
Vonesh JR (2005) Sequential predator effects across three life stages of the African tree frog, Hyperolius spinigularis. Oecologia 143:280–290. doi:10.1007/s00442-004-1806-x
Vonesh JR, Bolker BM (2005) Compensatory larval responses shift trade-offs associated with predator-induced hatching plasticity. Ecology 86:1580–1591. doi:10.1890/04-0535
Vonesh JR, Osenberg CW (2003) Multiple-predator effects across life-history stages: non-additive of egg- and larval-stage predation in an African treefrog. Ecol Lett 3:503–508. doi:10.1046/j.1461-0248.2003.00470.x
Vonesh JR, Warkentin KM (2006) Opposite shifts in size at metamorphosis in response to larval and metamorph predators. Ecology 87:556–562. doi:10.1890/05-0930
Warkentin KM (1995) Adaptative plasticity in hatching age: a response to predation risk trade-offs. Proc Natl Acad Sci USA 92:3507–3510. doi:10.1073/pnas.92.8.3507
Warkentin KM (1999) The development of behavioral defenses: a mechanistic analysis of vulnerability in red-eyed tree frog hatchlings. Behav Ecol 10:251–262. doi:10.1093/beheco/10.3.251
Wedekind C, Müller R (2005) Risk-induced early hatching in salmonids. Ecology 86:2525–2529. doi:10.1890/04-1738
Werner EE, Anholt BR (1993) Ecological consequences of the trade-off between growth and mortality rates mediated by foraging activity. Am Nat 142:242–272. doi:10.1086/285537
Wilbur HM, Collins JP (1973) Ecological aspects of amphibian metamorphosis. Science 182:1305–1314. doi:10.1126/science.182.4119.1305
Acknowledgments
We thank Felix González for valuable assistance in obtaining the experimental animals. An anonymous reviewer provided extremely helpful comments on the manuscript. We also thank Consejería de Medio Ambiente del Principado de Asturias and Consejería de Medio Ambiente de la Junta de Castilla y León for permissions to conduct this research. This work was funded by a MCYT research grant (REN2001-2647/GLO) to A.G. Nicieza. E.Capellán was supported by a FPU doctoral fellowship from MEC. The experiments conducted in this study complied with the institutional and country requirements on animal welfare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Capellán, E., Nicieza, A.G. Constrained plasticity in switching across life stages: pre- and post-switch predators elicit early hatching. Evol Ecol 24, 49–57 (2010). https://doi.org/10.1007/s10682-008-9289-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-008-9289-6