Abstract
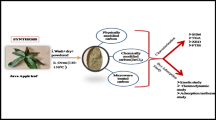
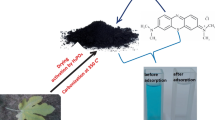
In the present study, activated carbon prepared from H2SO4-functionalized Moringa oleifera leaves (ACMOL) was used as a potential adsorbent for the effective removal of malachite green (MG) dye from aqueous media. FT-IR, SEM, EDS, Zeta potential, XRD, BET, proximate, and CHNS analysis techniques were used for surface characterization of the ACMOL. The adsorption efficiency of the ACMOL was investigated as a function of varying adsorbent dosage (0.02–0.2 g/100 mL), pH (3.0–9.0), ionic strength (0.1–0.5 M KCl), urea concentration (0.1–0.5 M), contact time (30–210 min), and temperature (303–323 K). The representative adsorption isotherms belong to the typical L-type. Maximum percentage removal was found to be 84% (124.40 mg/g) for MG dye concentration (30 mg/L) at pH 7.0 and 303 K with ACMOL dose 0.02 g/100 mL. The adsorption kinetics and equilibrium experimental data of MG dye adsorption on the ACMOL were well explained by the pseudo-second-order kinetics (R2 = 0.99) and Langmuir isotherm model (R2 = 0.99), respectively. The value of adsorption and desorption coefficient was found to be 0.036 min−1 and 0.025 mg min−1/L, respectively. Thermodynamic study showed the spontaneous (ΔG° = − 31.33, − 31.92, and − 32.49 kJ/mol at temperatures 303 K, 313 K, and 323 K, respectively) and exothermic (ΔH° = − 13.7 kJ/mol) nature of the adsorption with some structural changes occurring on the ACMOL surface (ΔS° = 58.198 J/K·mol).














Similar content being viewed by others
Data availability
It will be made available on reasonable request.
References
Abbas, M. (2020). Experimental investigation of activated carbon prepared from apricot stones material (ASM) adsorbent for removal of malachite green (MG) from aqueous solution. Adsorption Science & Technology, 38(1–2), 24–45. https://doi.org/10.1177/0263617420904476
Abdollahzadeh, H., Fazlzadeh, M., Afshin, S., Arfaeinia, H., Feizizadeh, A., Poureshgh, Y., & Rashtbari, Y. (2022). Efficiency of activated carbon prepared from scrap tires magnetized by Fe3O4 nanoparticles: Characterisation and its application for removal of reactive blue19 from aquatic solutions. International Journal of Environmental Analytical Chemistry, 102(8), 1911–1925. https://doi.org/10.1080/03067319.2020.1745199
Adeyi, A. A., Jamil, S. N., Abdullah, L. C., & Choong, T. S. (2019). Adsorption of malachite green dye from liquid phase using hydrophilic thiourea-modified poly (acrylonitrile-co-acrylic acid): Kinetic and isotherm studies. Journal of Chemistry. https://doi.org/10.1155/2019/4321475
Adeyi, A. A., Jamil, S. N. A. M., Abdullah, L. C., Choong, T. S. Y., Lau, K. L., & Alias, N. H. (2020). Simultaneous adsorption of malachite green and methylene blue dyes in a fixed-bed column using poly (acrylonitrile-co-acrylic acid) modified with thiourea. Molecules, 25(11), 26–50. https://doi.org/10.3390/molecules25112650
Ahmad, A. A., Ahmad, M. A., Yahaya, N. K. E., & Karim, J. (2021). Adsorption of malachite green by activated carbon derived from gasified Hevea brasiliensis root. Arabian Journal of Chemistry, 14(4), 103104. https://doi.org/10.1016/j.arabjc.2021.103104
Ahmadfazeli, A., Poureshgh, Y., Rashtbari, Y., Akbari, H., Pourali, P., & Adibzadeh, A. (2021). Removal of metronidazole antibiotic from aqueous solution by ammonia-modified activated carbon: Adsorption isotherm and kinetic study. Journal of Water, Sanitation and Hygiene for Development, 11(6), 1083–1096.
Al-Ghouti, M. A., Khraisheh, M. A. M., Allen, S. J., & Ahmad, M. N. (2003). The removal of dyes from textile wastewater: A study of the physical characteristics and adsorption mechanisms of diatomaceous earth. Journal of Environmental Management, 69(3), 229–238. https://doi.org/10.1016/j.jenvman.2003.09.005
Anjorin, T. S., Ikokoh, P., & Okolo, S. (2010). Mineral composition of Moringa oleifera leaves, pods and seeds from two regions in Abuja, Nigeria. International Journal of Agriculture and Biology, 12(3), 431–434.
Askari, R., Afshin, S., Rashtbari, Y., Moharrami, A., Mohammadi, F., Vosuoghi, M., & Dargahi, A. (2023). Synthesis of activated carbon from walnut wood and magnetized with cobalt ferrite (CoFe2O4) and its application in removal of cephalexin from aqueous solutions. Journal of Dispersion Science and Technology, 44(7), 1183–1194. https://doi.org/10.1080/01932691.2021.2008421
Banerjee, S., & Chattopadhyaya, M. C. (2017). Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low-cost agricultural by-product. Arabian Journal of Chemistry, 10, S1629–S1638. https://doi.org/10.1016/j.arabjc.(2013).06.005
Bekçi, Z., Seki, Y., & Cavas, L. (2009). Removal of malachite green by using an invasive marine alga Caulerpa racemosa var. cylindracea. Journal of hazardous materials, 161(2–3), 1454–1460. https://doi.org/10.1016/j.jhazmat.2008.04.125
Bello, O. S. (2013). Adsorptive removal of malachite green with activated carbon prepared from oil palm fruit fibre by KOH activation and CO2 gasification. South African Journal of Chemistry, 66, 32–41. https://doi.org/10.3390/molecules25112650
Bello, O. S., & Ahmad, M. A. (2011). Adsorptive removal of a synthetic textile dye using cocoa pod husks. Toxicological & Environmental Chemistry, 93(7), 1298–1308. https://doi.org/10.1080/02772248.2011.590490
Bhomick, P. C., Supong, A., Kumar, S., Sema, A. I., Merry, T., & Sinha, D. (2023). Utilization of Pinus kesiya and Schima wallichii biomass-derived activated carbon for methylene blue removal: Adsorption performance and mechanistic insights. Water Conservation Science and Engineering, 8(1), 1–15. https://doi.org/10.1007/s41101-023-00220-0
Choudhary, M., Kumar, R., & Neogi, S. (2020). Activated biochar derived from Opuntia ficus-indica for the efficient adsorption of malachite green dye, Cu+ 2 and Ni+ 2 from water. Journal of Hazardous Materials, 392, 122441. https://doi.org/10.1016/j.jhazmat.2020.122441
Chowdhury, S., Mishra, R., Saha, P., & Kushwaha, P. (2011). Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination, 265(1–3), 159–168. https://doi.org/10.1016/j.desal.2010.07.047
Ciğeroğlu, Z., Kazan-Kaya, E. S., El Messaoudi, N., Fernine, Y., Americo-Pinheiro, J. H. P., & Jada, A. (2023). Remediation of tetracycline from aqueous solution through adsorption on g-C3N4-ZnO-BaTiO3 nanocomposite: Optimization, modeling, and theoretical calculation. Journal of Molecular Liquids, 369, 120866. https://doi.org/10.1016/j.molliq.2022.120866
Dahri, M. K., Kooh, M. R. R., & Lim, L. B. (2014). Water remediation using low cost adsorbent walnut shell for removal of malachite green: Equilibrium, kinetics, thermodynamic and regeneration studies. Journal of Environmental Chemical Engineering, 2(3), 1434–1444. https://doi.org/10.1016/j.jece.2014.07.008
Danial, R., Sobri, S., Abdullah, L. C., & Mobarekeh, M. N. (2019). FTIR, CHNS and XRD analyses define mechanism of glyphosate herbicide removal by electrocoagulation. Chemosphere, 233, 559–569. https://doi.org/10.1016/j.chemosphere.2019.06.010
Debord, J., Harel, M., Bollinger, J. C., & Chu, K. H. (2022). The Elovich isotherm equation: Back to the roots and new developments. Chemical Engineering Science, 262, 118012. https://doi.org/10.1016/j.ces.2022.118012
El Khomri, M., El Messaoudi, N., Dbik, A., Bentahar, S., Lacherai, A., Chegini, Z. G., & Bouich, A. (2021). Removal of Congo red from aqueous solution in single and binary mixture systems using Argan nutshell wood. Pigment & Resin Technology, 51(5), 477–488. https://doi.org/10.1108/PRT-04-2021-0045
El Khomri, M., El Messaoudi, N., Dbik, A., Bentahar, S., Fernine, Y., Lacherai, A., & Jada, A. (2022). Optimization based on response surface methodology of anionic dye desorption from two agricultural solid wastes. Chemistry Africa, 5(4), 1083–1095. https://doi.org/10.1007/s42250-022-00395-4
El Messaoudi, N., Dbik, A., El Khomri, M., Sabour, A., Bentahar, S., & Lacherai, A. (2017a). Date stones of Phoenix dactylifera and jujube shells of Ziziphus lotus as potential biosorbents for anionic dye removal. International Journal of Phytoremediation, 19(11), 1047–1052. https://doi.org/10.1080/15226514.2017.1319331
El Messaoudi, N., El Khomri, M., Dbik, A., Bentahar, S., & Lacherai, A. (2017b). Selective and competitive removal of dyes from binary and ternary systems in aqueous solutions by pretreated jujube shell (Zizyphus lotus). Journal of Dispersion Science and Technology, 38(8), 1168–1174. https://doi.org/10.1080/01932691.2016.1228070
El Messaoudi, N., El Khomri, M., Chegini, Z. G., Bouich, A., Dbik, A., Bentahar, S., Labjar, N., Iqbal, M., Jada, A., & Lacherai, A. (2022a). Dye removal from aqueous solution using nanocomposite synthesized from oxalic acid-modified agricultural solid waste and ZnFe 2 O 4 nanoparticles. Nanotechnology for Environmental Engineering, 7, 1–15. https://doi.org/10.1007/s41204-021-00173-6
El Messaoudi, N., El Khomri, M., Chegini, Z. G., Dbik, A., Bentahar, S., Iqbal, M., Jada, A., & Lacherai, A. (2022b). Desorption of crystal violet from alkali-treated agricultural material waste: An experimental study, kinetic, equilibrium and thermodynamic modeling. Pigment & Resin Technology, 51(3), 309–319. https://doi.org/10.1108/PRT-02-2021-0019
El Messaoudi, N., El Mouden, A., El Khomri, M., Bouich, A., Fernine, Y., Ciğeroğlu, Z., Americo-Pinheiro, J. H. P., Labjar, N., Jada, A., Sillanpää, M., & Lacherai, A. (2023). Experimental study and theoretical statistical modeling of acid blue 25 remediation using activated carbon from Citrus sinensis leaf. Fluid Phase Equilibria, 563, 113585. https://doi.org/10.1016/j.fluid.2022.113585
El Mouden, A., El Guerraf, A., El Messaoudi, N., Haounati, R., Ait El Fakir, A., & Lacherai, A. (2022). Date stone functionalized with 3-aminopropyltriethoxysilane as a potential biosorbent for heavy metal ions removal from aqueous solution. Chemistry Africa, 5(3), 745–759. https://doi.org/10.1007/s42250-022-00350-3
El Messaoudi, N., El Khomri, M., Goodarzvand Chegini, Z., Chlif, N., Dbik, A., Bentahar, S., Iqbal, M., Jada, A., & Lacherai, A. (2021). Desorption study and reusability of raw and H2SO4 modified jujube shells (Zizyphus lotus) for the methylene blue adsorption. International Journal of Environmental Analytical Chemistry, 1–17. https://doi.org/10.1080/03067319.2021.1912338
El Messaoudi, N., El Khomri, M., El Mouden, A., Bouich, A., Jada, A., Lacherai, A., Iqbal, H. M., Mulla, S. I., Kumar, V., & Américo-Pinheiro, J. H. P. (2022). Regeneration and reusability of non-conventional low-cost adsorbents to remove dyes from wastewaters in multiple consecutive adsorption–desorption cycles: A review. Biomass Conversion and Biorefinery, 1–18. https://doi.org/10.1007/s13399-022-03604-9
Freundlich, H. M. F. (1906). Over the adsorption in solution. Journal of Physical Chemistry, 57, 385–471.
Gedam, V. V., Raut, P., Chahande, A., & Pathak, P. (2019). Kinetic, thermodynamics and equilibrium studies on the removal of Congo red dye using activated teak leaf powder. Applied Water Science, 9, 1–13. https://doi.org/10.1007/s13201-019-0933-9
Ghaedi, M., Ansari, A., & Sahraei, R. (2013). ZnS: Cu nanoparticles loaded on activated carbon as novel adsorbent for kinetic, thermodynamic and isotherm studies of Reactive Orange 12 and Direct yellow 12 adsorption. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 114, 687–694. https://doi.org/10.1016/j.saa.2013.04.091
Gündüz, F., & Bayrak, B. (2017). Biosorption of malachite green from an aqueous solution using pomegranate peel: Equilibrium modelling, kinetic and thermodynamic studies. Journal of Molecular Liquids, 243, 790–798. https://doi.org/10.1016/j.molliq.2017.08.095
Gupta, V. K., Jain, R., Mittal, A., Saleh, T. A., Nayak, A., Agarwal, S., & Sikarwar, S. (2012). Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions. Materials Science and Engineering: C, 32(1), 12–17. https://doi.org/10.1016/j.msec.2011.08.018
Gupta, N., Kushwaha, A. K., & Chattopadhyaya, M. C. (2016). Application of potato (Solanum tuberosum) plant wastes for the removal of methylene blue and malachite green dye from aqueous solution. Arabian Journal of Chemistry, 9, S707–S716. https://doi.org/10.1016/j.arabjc.2011.07.021
Hamdaoui, O. (2006). Batch study of liquid-phase adsorption of methylene blue using cedar sawdust and crushed brick. Journal of Hazardous Materials, 135(1–3), 264–273. https://doi.org/10.1016/j.jhazmat.2005.11.062
Hameed, K. S., Muthirulan, P., & Sundaram, M. M. (2017). Adsorption of chromotrope dye onto activated carbons obtained from the seeds of various plants: Equilibrium and kinetics studies. Arabian Journal of Chemistry, 10, S2225–S2233. https://doi.org/10.1016/j.arabjc.2013.07.058
Jabar, J. M., & Odusote, Y. A. (2021). Utilization of prepared activated biochar from water lily (Nymphaea lotus) stem for adsorption of malachite green dye from aqueous solution. Biomass Conversion and Biorefinery, 1–12. https://doi.org/10.1007/s13399-021-01399-9
Jiang, D., Li, H., Cheng, X., Ling, Q., Chen, H., Barati, B., Yao, Q., Abomohra, A., Hu, X., Bartocci, P., & Wang, S. (2023). A mechanism study of methylene blue adsorption on seaweed biomass derived carbon: From macroscopic to microscopic scale. Process Safety and Environmental Protection, 172, 1132–1143. https://doi.org/10.1016/j.psep.2023.02.044
Kalaiyan, G., Suresh, S., Prabu, K. M., Thambidurai, S., Kandasamy, M., Pugazhenthiran, N., Kumar, S. K., & Muneeswaran, T. (2021). Bactericidal activity of Moringa oleifera leaf extract assisted green synthesis of hierarchical copper oxide microspheres against pathogenic bacterial strains. Journal of Environmental Chemical Engineering, 9(1), 104847. https://doi.org/10.1016/j.jece.2020.104847
Kansal, S. K., & Kumari, A. (2014). Potential of M. oleifera for the treatment of water and wastewater. Chemical reviews, 114(9), 4993–5010. https://doi.org/10.1021/cr400093w
Kehrein, P., Van Loosdrecht, M., Osseweijer, P., Garfí, M., Dewulf, J., & Posada, J. (2020). A critical review of resource recovery from municipal wastewater treatment plants–market supply potentials, technologies and bottlenecks. Environmental Science: Water Research & Technology, 6(4), 877–910. https://doi.org/10.1039/C9EW00905A
Khalid, S., Shahid, M., Natasha, Bibi, I., Sarwar, T., Shah, A. H., & Niazi, N. K. (2018). A review of environmental contamination and health risk assessment of wastewater use for crop irrigation with a focus on low and high-income countries. International Journal of Environmental Research and Public Health, 15(5), 895. https://doi.org/10.3390/ijerph15050895
Khattri, S. D., & Singh, M. K. (2009). Removal of malachite green from dye wastewater using neem sawdust by adsorption. Journal of Hazardous Materials, 167(1–3), 1089–1094. https://doi.org/10.1016/j.jhazmat.2009.01.101
Khomri, M. E., Messaoudi, N. E., Dbik, A., Bentahar, S., Fernine, Y., Bouich, A., Lacherai, A., & Jada, A. (2022). Modification of low-cost adsorbent prepared from agricultural solid waste for the adsorption and desorption of cationic dye. Emergent Materials, 5(6), 1679–1688. https://doi.org/10.1007/s42247-022-00390-y
Kooh, M. R. R., Dahri, M. K., & Lim, L. B. (2017). Removal of methyl violet 2B dye from aqueous solution using Nepenthes rafflesiana pitcher and leaves. Applied Water Science, 7, 3859–3868. https://doi.org/10.1007/s13201-017-0537-1
Kooh, M. R. R., Dahri, M. K., Lim, L. B., Lim, L. H., & Chan, C. M. (2018). Separation of acid blue 25 from aqueous solution using water lettuce and agro-wastes by batch adsorption studies. Applied Water Science, 8, 1–10. https://doi.org/10.1007/s13201-018-0714-x
Kumar, K. V., & Porkodi, K. (2007). Batch adsorber design for different solution volume/adsorbent mass ratios using the experimental equilibrium data with fixed solution volume/adsorbent mass ratio of malachite green onto orange peel. Dyes and Pigments, 74(3), 590–594. https://doi.org/10.1016/j.dyepig.2006.03.024
Kundu, S., & Gupta, A. K. (2006). Arsenic adsorption onto iron oxide-coated cement (IOCC): Regression analysis of equilibrium data with several isotherm models and their optimization. Chemical Engineering Journal, 122(1–2), 93–106. https://doi.org/10.1016/j.cej.2006.06.002
Lehmann, S. (2012). Can rapid urbanisation ever lead to low carbon cities? The case of Shanghai in comparison to Potsdamer Platz Berlin. Sustainable Cities and Society, 3, 1–12. https://doi.org/10.1016/j.scs.2011.08.001
Li, H., Kong, J., Zhang, H., Gao, J., Fang, Y., Shi, J., Ge, T., Fang, T., Shi, Y., Zhang, R., & Zhang, N. (2023). Mechanisms and adsorption capacities of ball milled biomass fly ash/biochar composites for the adsorption of methylene blue dye from aqueous solution. Journal of Water Process Engineering, 53, 103713. https://doi.org/10.1016/j.jwpe.2023.103713
Lima, E. C., Hosseini-Bandegharaei, A., Moreno-Piraján, J. C., & Anastopoulos, I. (2019). A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. Journal of Molecular Liquids, 273, 425–434. https://doi.org/10.1016/J.MOLLIQ.2018.10.048
Lu, Y. C., Kooh, M. R. R., Lim, L. B. L., & Priyantha, N. (2021). Effective and simple NaOH-modification method to remove methyl violet dye via Ipomoea aquatica roots. Adsorption Science & Technology, 2021, 1–12. https://doi.org/10.1155/2021/5932222
Mamuad, R. Y., Pascual, M. F. T., & Choi, A. E. S. (2022). Development of a low-cost dispenser-type water filtration system. Cleaner and Responsible Consumption, 7, 100085. https://doi.org/10.1016/j.clrc.2022.100085
Mashkoor, F., & Nasar, A. (2019). Preparation, characterization and adsorption studies of the chemically modified Luffa aegyptica peel as a potential adsorbent for the removal of malachite green from aqueous solution. Journal of Molecular Liquids, 274, 315–327. https://doi.org/10.1016/j.molliq.2018.10.119
Mishra, P., Singh, K., & Dixit, U. (2021). Adsorption, kinetics and thermodynamics of phenol removal by ultrasound-assisted sulfuric acid-treated pea (Pisum sativum) shells. Sustainable Chemistry and Pharmacy, 22, 100491. https://doi.org/10.1016/j.scp.2021.100491
Mishra, P., Singh, K., Dixit, U., Agarwal, A., & Bhat, R. A. (2022a). Effective removal of 4-Aminophenol from aqueous environment by pea (Pisum sativum) shells activated with sulfuric acid: Characterization, isotherm, kinetics and thermodynamics. Journal of the Indian Chemical Society, 99(7), 100528. https://doi.org/10.1016/j.jics.2022.100528
Mishra, P., Singh, K., & Pandey, G. (2022b). A comparative study of phenol removal by Pisum-sativum peels biochars derived at different pyrolysis temperatures: Isotherm, kinetic andthermodynamicmodelling. ChemistrySelect, 7(39), e202202856. https://doi.org/10.1002/slct.202202856
Mittal, A., Mittal, J., Malviya, A., & Gupta, V. K. (2009). Adsorptive removal of hazardous anionic dye “Congo red” from wastewater using waste materials and recovery by desorption. Journal of Colloid and Interface Science, 340(1), 16–26. https://doi.org/10.1016/j.jcis.2009.08.019
Mittal, A., Mittal, J., Malviya, A., & Gupta, V. K. (2010). Removal and recovery of Chrysoidine Y from aqueous solutions by waste materials. Journal of Colloid and Interface Science, 344(2), 497–507. https://doi.org/10.1016/j.jcis.2010.01.007
Munagapati, V. S., Yarramuthi, V., Kim, Y., Lee, K. M., & Kim, D. S. (2018). Removal of anionic dyes (Reactive Black 5 and Congo Red) from aqueous solutions using Banana Peel Powder as an adsorbent. Ecotoxicology and Environmental Safety, 148, 601–607. https://doi.org/10.1016/j.ecoenv.2017.10.075
Natarajan, S., Bajaj, H. C., & Tayade, R. J. (2018). Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process. Journal of Environmental Sciences, 65, 201–222. https://doi.org/10.1016/j.jes.2017.03.011
Piriya, R. S., Jayabalakrishnan, R. M., Maheswari, M., Boomiraj, K., & Oumabady, S. (2023). Comparative adsorption study of malachite green dye on acid-activated carbon. International Journal of Environmental Analytical Chemistry, 103(1), 16–30. https://doi.org/10.1080/03067319.2020.1849667
Podstawczyk, D., Witek-Krowiak, A., Chojnacka, K., & Sadowski, Z. (2014). Biosorption of malachite green by eggshells: Mechanism identification and process optimization. Bioresource Technology, 160, 161–165. https://doi.org/10.1016/j.biortech.2014.01.015
Rajeshkannan, R., Rajasimman, M., & Rajamohan, N. (2011). Decolourization of malachite green using tamarind seed: Optimization, isotherm and kinetic studies. Chemical Industry and Chemical Engineering Quarterly/CICEQ, 17(1), 67–79. https://doi.org/10.2298/CICEQ100716056R
Rashtbari, Y., Américo-Pinheiro, J. H. P., Bahrami, S., Fazlzadeh, M., Arfaeinia, H., & Poureshgh, Y. (2020). Efficiency of zeolite coated with zero-valent iron nanoparticles for removal of humic acid from aqueous solutions. Water, Air, & Soil Pollution, 231(10), 514. https://doi.org/10.1007/s11270-020-04872-9
Rashtbari, Y., Arfaeinia, H., Ahmadi, S., BahramiAsl, F., Afshin, S., Poureshgh, Y., & Fazlzadeh, M. (2022b). Potential of using green adsorbent of humic acid removal from aqueous solutions: Equilibrium, kinetics, thermodynamic and regeneration studies. International Journal of Environmental Analytical Chemistry, 102(17), 5373–5390. https://doi.org/10.1080/03067319.2020.1796993
Rashtbari, Y., Abazari, M., Arfaeinia, L., Gholizadeh, A., Afshin, S., Poureshgh, Y., & Alipour, M. (2023). The optimization of reactive black 5 dye removal in the sono-catalytic process combined with local yellow montmorillonite and hydrogen peroxide using response surface methodology from aqueous solutions. Biomass Conversion and Biorefinery, 13(7), 6067–6081. https://doi.org/10.1007/s13399-021-01773-7
Rashtbari, Y., Afshin, S., Hamzezadeh, A., Gholizadeh, A., Ansari, F. J., Poureshgh, Y., & Fazlzadeh, M. (2022). Green synthesis of zinc oxide nanoparticles loaded on activated carbon prepared from walnut peel extract for the removal of Eosin Y and Erythrosine B dyes from aqueous solution: Experimental approaches, kinetics models, and thermodynamic studies. Environmental Science and Pollution Research, 1–13. https://doi.org/10.1007/s11356-021-16006-7
Sadegh, N., Haddadi, H., Arabkhani, P., Asfaram, A., & Sadegh, F. (2021). Simultaneous elimination of rhodamine B and malachite green dyes from the aqueous sample with magnetic reduced graphene oxide nanocomposite: Optimization using experimental design. Journal of Molecular Liquids, 343, 117710. https://doi.org/10.1016/j.molliq.2021.117710
Saha, P., Chowdhury, S., Gupta, S., Kumar, I., & Kumar, R. (2010). Assessment on the removal of malachite green using tamarind fruit shell as biosorbent. CLEAN–Soil, Air, Water, 38(5–6), 437–445. https://doi.org/10.1002/clen.200900234
Salem, M. Z., Ali, H. M., & Akrami, M. (2021). Moringa oleifera seeds-removed ripened pods as alternative for papersheet production: Antimicrobial activity and their phytoconstituents profile using HPLC. Scientific Reports, 11(1), 19027. https://doi.org/10.1038/s41598-021-98415-9
Salleh, M. A. M., Mahmoud, D. K., Karim, W. A. W. A., & Idris, A. (2011). Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination, 280(1–3), 1–13. https://doi.org/10.1016/j.desal.2011.07.019
Sassi, W., Ghanmi, I., Oulego, P., Collado, S., Ammar, S., & Díaz, M. (2023). Pomegranate peel-derived biochar as ecofriendly adsorbent of aniline-based dyes removal from wastewater. Clean Technologies and Environmental Policy, 1–17. https://doi.org/10.1007/s10098-023-02522-2
Sekhar, C. P., Kalidhasan, S., Rajesh, V., & Rajesh, N. (2009). Bio-polymer adsorbent for the removal of malachite green from aqueous solution. Chemosphere, 77(6), 842–847. https://doi.org/10.1016/j.chemosphere.2009.07.068
Şenol, Z. M., Messaoudi, N. E., Fernine, Y., & Keskin, Z. S. (2023). Bioremoval of rhodamine B dye from aqueous solution by using agricultural solid waste (almond shell): Experimental and DFT modeling studies. Biomass Conversion and Biorefinery, 1–14. https://doi.org/10.1007/s13399-023-03781-1
Sharma, Y. C. (2009). Fast removal of malachite green by adsorption on rice husk activated carbon. The Open Environmental Pollution & Toxicology Journal, 1(1). https://doi.org/10.2174/1876397900901010074
Shokoohi, R., Samadi, M. T., Amani, M., & Poureshgh, Y. (2018). Modeling and optimization of removal of cefalexin from aquatic solutions by enzymatic oxidation using experimental design. Brazilian Journal of Chemical Engineering, 35, 943–956. https://doi.org/10.1590/0104-6632.20180353s20170383
Simonin, J. P. (2016). On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chemical Engineering Journal, 300, 254–263. https://doi.org/10.1016/j.cej.2016.04.079
Singh, K., & Mohan, S. (2004). Adsorption behavior of selected monosaccharides onto an alumina interface. Journal of Colloid and Interface Science, 270(1), 21–28. https://doi.org/10.1016/j.jcis.2003.05.002
Singh, K., Bharose, R., Singh, V. K., & Verma, S. K. (2011). Sugar decolorization through selective adsorption onto functionalized Accurel hydrophobic polymeric support. Industrial & Engineering Chemistry Research, 50(17), 10074–10082. https://doi.org/10.1021/ie200501p
Singh, K., Kumar, A., Awasthi, S., Pandey, S. K., & Mishra, P. (2019). Adsorption mechanism of carboxymethyl cellulose onto mesoporous mustard carbon: Experimental and theoretical aspects. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 581, 123786. https://doi.org/10.1016/j.colsurfa.2019.123786
Singh, K., Dixit, U., & Lata, M. (2023). Surface activity, kinetics, thermodynamics and comparative study of adsorption of selected cationic and anionic dyes onto H3PO4-functionalized bagasse from aqueous stream. Environmental Science and Pollution Research, 1–17. https://doi.org/10.1007/s11356-023-29870-2
Srivastava, S., Sinha, R., & Roy, D. (2004). Toxicological effects of malachite green. Aquatic Toxicology, 66(3), 319–329. https://doi.org/10.1016/j.aquatox.2003.09.008
Suhaimi, N., Kooh, M. R. R., Lim, C. M., Chou Chao, C. T., Chou Chau, Y. F., Mahadi, A. H., Chiang, H. P., Haji Hassan, N. H., & Thotagamuge, R. (2022). The use of gigantochloa bamboo-derived biochar for the removal of methylene blue from aqueous solution. Adsorption Science & Technology, 2022, 1–12. https://doi.org/10.1155/2022/8245797
Swan, N. B., & Zaini, M. A. A. (2019). Adsorption of malachite green and congo red dyes from water: Recent progress and future outlook. Ecological Chemistry and Engineering, 26(1), 119–132.
Türgay, O., Ersöz, G., Atalay, S., Forss, J., & Welander, U. (2011). The treatment of azo dyes found in textile industry wastewater by anaerobic biological method and chemical oxidation. Separation and Purification Technology, 79(1), 26–33. https://doi.org/10.1016/j.biortech.2013.07.066
Vigneshwaran, S., Sirajudheen, P., Karthikeyan, P., & Meenakshi, S. (2021). Fabrication of sulfur-doped biochar derived from tapioca peel waste with superior adsorption performance for the removal of Malachite green and Rhodamine B dyes. Surfaces and Interfaces, 23, 100920. https://doi.org/10.1016/j.surfin.2020.100920
Vijayalakshmidevi, S. R., & Muthukumar, K. (2015). Improved biodegradation of textile dye effluent by coculture. Ecotoxicology and Environmental Safety, 114, 23–30. https://doi.org/10.1016/j.ecoenv.2014.09.039
Vijayaraghavan, K. T. V. N., Padmesh, T. V. N., Palanivelu, K., & Velan, M. (2006). Biosorption of nickel (II) ions onto Sargassum wightii: Application of two-parameter and three-parameter isotherm models. Journal of Hazardous Materials, 133, 304–308. https://doi.org/10.1016/j.jhazmat.2005.10.016
Wang, X. S., Zhou, Y., Jiang, Y., & Sun, C. (2008). The removal of basic dyes from aqueous solutions using agricultural by-products. Journal of Hazardous Materials, 157(2–3), 374–385. https://doi.org/10.1016/j.jhazmat.2008.01.004
**e, Z., Guan, W., Ji, F., Song, Z., & Zhao, Y. (2014). Production of biologically activated carbon from orange peel and landfill leachate subsequent treatment technology. Journal of Chemistry, 2014. https://doi.org/10.1155/2014/491912
**ng, Y., & Wang, G. (2009). Poly (methacrylic acid)-modified sugarcane bagasse for enhanced adsorption of cationic dye. Environmental Technology, 30(6), 611–619. https://doi.org/10.1080/09593330902838098
**ong, C., Jia, Q., Chen, X., Wang, G., & Yao, C. (2013). Optimization of polyacrylonitrile-2-aminothiazole resin synthesis, characterization, and its adsorption performance and mechanism for removal of Hg (II) from aqueous solutions. Industrial & Engineering Chemistry Research, 52(14), 4978–4986. https://doi.org/10.1021/ie3033312
**ong, C., Zheng, Y., Feng, Y., Yao, C., Ma, C., Zheng, X., & Jiang, J. (2014). Preparation of a novel chloromethylated polystyrene-2-amino-1, 3, 4-thiadiazole chelating resin and its adsorption properties and mechanism for separation and recovery of Pt (IV) from aqueous solutions. Journal of Materials Chemistry A, 2(15), 5379–5386. https://doi.org/10.1039/C3TA14923D
Yıldız, D., Demir, I., & Demiral, H. (2023). Adsorption of malachite green on to poplar sawdust activated carbon. Separation Science and Technology, 58(12), 2099–2114. https://doi.org/10.1080/01496395.2023.2240492
شکوهی, ص., صمدی, امانی, & پورعشق, (2018). Optimizing laccase-mediated amoxicillin removal by the use of box–behnken design in an aqueous solution. Desalination and Water Treatment, 119, 53-63.
Acknowledgements
The authors also acknowledge the National Sugar Institute, Kanpur, for providing some experimental facilities. Utkarsh Dixit is grateful to UGC, New Delhi, for its financial support (UGC-JRF-191620022543).
Author information
Authors and Affiliations
Contributions
Utkarsh Dixit: conceptualization, investigation, validation, methodology, writing—original draft. Kaman Singh: conceptualization, investigation, validation, methodology, supervision, data curation, review and editing. Sudhanshu Mohan: conceptualization, investigation, validation, methodology, supervision. Alok Kumar Singh: data curation, writing—original draft. Arun Kumar: data curation, writing—original draft.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
All authors have read and understood and have complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for Authors. All the authors have their consent in submitting the manuscript to the journal “Environmental Monitoring and Assessment.”
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dixit, U., Singh, K., Mohan, S. et al. Surface activity, mechanisms, kinetics, and thermodynamic study of adsorption of malachite green dye onto sulfuric acid–functionalized Moringa oleifera leaves from aqueous solution. Environ Monit Assess 196, 78 (2024). https://doi.org/10.1007/s10661-023-12234-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-12234-1