Summary
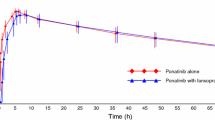
To evaluate the potential gastric pH-dependent drug-drug interaction (DDI), safety and tolerability of famitinib co-administered with omeprazole in healthy subjects. Twenty healthy subjects were enrolled in a single-center, single-arm, open-label, fixed-sequence study. Famitinib was administered as a single oral 25 mg under a fasting condition on day 1, omeprazole (40 mg once daily) was given on days 10–14, concomitantly with famitinib on day 15, and for the follow-up 7 additional days (days 16–22). Blood samples were collected for the pharmacokinetic analysis of famitinib and its metabolite SHR116637 following each famitinib dose. Safety and tolerability were assessed during the whole progress via clinical laboratory tests. The least-squares geometric mean ratios (GMRs) (90% CI) of Cmax, AUC0-t and AUC0-∞ for famitinib combined with omeprazole to famitinib alone were 0.989 (0.953, 1.027), 0.956 (0.907, 1.007) and 0.953(0.905, 1.005) respectively. For the metabolite SHR116637, their GMRs (90% CI) of the above parameters were 0.851 (0.786, 0.920), 0.890 (0.838, 0.946)and 0.887 (0.835, 0.943), indicating the absence of significant differences in the parameters. During the treatment period, 9(45%) subjects reported 16 treatment emergent adverse events (TEAE), among which 6 subjects (30%) reported 9 TEAEs and 1 subject (5%) reported 1 TEAE during famitinib or omeprazole administered alone respectively, 5 subjects (25.0%) reported 6 TEAEs during in the combined administration phase. Omeprazole did not have a significant influence on the pharmacokinetics (PK) of famitinib and SHR116637, and the safety profile was good upon co-administration. ClinicalTrials.gov identifier NCT 05,041,920.



Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Raoul JL, Edeline J, Simmet V, Moreau-Bachelard C, Gilabert M, Frenel JS (2022) Long-Term Use of Proton Pump Inhibitors in Cancer Patients: An Opinion Paper. Cancers (Basel) 14(5).https://doi.org/10.3390/cancers14051156
Zhang L, Wu F, Lee SC, Zhao H, Zhang L (2014) pH-dependent drug-drug interactions for weak base drugs: potential implications for new drug development. Clin Pharmacol Ther 96(2):266–277.https://doi.org/10.1038/clpt.2014.87
Herbrink M, Nuijen B, Schellens JH, Beijnen JH (2015) Variability in bioavailability of small molecular tyrosine kinase inhibitors. Cancer Treat Rev 41(5):412–422.https://doi.org/10.1016/j.ctrv.2015.03.005
Kletzl H, Giraudon M, Ducray PS, Abt M, Hamilton M, Lum BL (2015) Effect of gastric pH on erlotinib pharmacokinetics in healthy individuals: omeprazole and ranitidine. Anticancer Drugs 26(5):565–572.https://doi.org/10.1097/CAD.0000000000000212
Pape E, Michel D, Scala-Bertola J, Schiestel T, Harle A, Bouchet S, Contet A, Pochon C, Gambier N (2016) Effect of esomeprazole on the oral absorption of dasatinib in a patient with Philadelphia-positive acute lymphoblastic leukemia. Br J Clin Pharmacol 81(6):1195–1196.https://doi.org/10.1111/bcp.12895
Zhou A, Zhang W, Chang C, Chen X, Zhong D, Qin Q, Lou D, Jiang H, Wang J (2013) Phase I study of the safety, pharmacokinetics and antitumor activity of famitinib. Cancer Chemother Pharmacol 72(5):1043–1053. https://doi.org/10.1007/s00280-013-2282-y
Mu X, Ma J, Zhang Z, Zhou H, Xu S, Qin Y, Huang J, Yang K, Wu G (2015) Famitinib enhances nasopharyngeal cancer cell radiosensitivity by attenuating radiation-induced phosphorylation of platelet-derived growth factor receptor and c-kit and inhibiting microvessel formation. Int J Radiat Biol 91(9):771–776.https://doi.org/10.3109/09553002.2015.1062574
Zhang W, Zhou AP, Qin Q, Chang CX, Jiang HY, Ma JH, Wang JW (2013) Famitinib in metastatic renal cell carcinoma: a single center study. Chin Med J (Engl) 126(22):4277–4281
Qu YY, Zhang HL, Guo H, Luo H, Zou Q, ** Wang
Contributions
Linlin Hu and Hui** Wang contributed to the study conception and design. Material preparation, data collection and analysis were performed by Linlin Hu, Mingmin Cai, Wei Qian, Lu Tang, Qiuyue Sun and Ting Dou. The first draft of the manuscript was written by Linlin Hu and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Research involving human participants and/or animals
This study was performed in line with the principles of the Declaration of Helsinki. The study protocol was approved (approval number, 2021ZDSYLL195-P01) by the Ethics Committee of the Zhongda Hospital, Medical School, Southeast University (Nan**g, China).
Conflict of interest statement
The authors declare no conflicts of interest. The author reports no conflicts of interest in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, L., Cai, M., Qian, W. et al. Phase I study to evaluate of the gastric pH-dependent drug interaction between famitinib and the proton pump inhibitor omeprazole in healthy subjects. Invest New Drugs 40, 1274–1281 (2022). https://doi.org/10.1007/s10637-022-01299-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-022-01299-3