Abstract
Purpose
Biallelic variants in POC1B are rare causes of autosomal recessive cone dystrophy associated with generalized cone system dysfunction. In this report, we describe the clinical characteristics of a Japanese male patient with POC1B-associated retinopathy with relatively preserved cone system function.
Methods
We performed whole-exome sequencing (WES) to identify the disease-causing variants and a comprehensive ophthalmic examination, including full-field and multifocal electroretinography (ffERG and mfERG).
Results
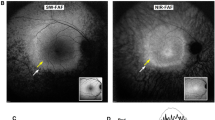
Our WES analysis identified novel compound heterozygous POC1B variants (p.Arg106Gln and p.Arg452Ter) in the patient. His unaffected mother carried the p.Arg452Ter variant heterozygously. The patient experienced decreased visual acuity in his 50s. At the age of 63, his corrected visual acuity was 20/22 in the right and 20/20 in the left eye. Fundus and fundus autofluorescence images for each eye showed no remarkable finding, except for a subtle hyperautofluorescent spot in the fovea of the left eye. Cross-sectional optical coherence tomography demonstrated blurred but a relatively preserved ellipsoid zone. The ffERG showed that amplitudes of rod and standard-flash responses were within the reference range, whereas the cone and light-adapted 30-Hz flicker amplitudes were close to, or slightly below, the reference range. The mfERG revealed substantially reduced responses with relative preservation of central function.
Conclusions
We reported the case of an older patient with POC1B-associated retinopathy, demonstrating late-onset visual decrease, good visual acuity, and relatively preserved cone system function. The disease condition was much milder than previously reported in patients with POC1B-associated retinopathy.







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Roosing S, Thiadens AA, Hoyng CB, Klaver CC, den Hollander AI, Cremers FP (2014) Causes and consequences of inherited cone disorders. Prog Retin Eye Res 42:1–26. https://doi.org/10.1016/j.preteyeres.2014.05.001
Tsang SH, Sharma T (2018) Progressive cone dystrophy and cone-rod dystrophy (XL, AD, and AR). In: Tsang SH, Sharma T (eds) Atlas of Inherited retinal diseases. Advances in experimental medicine and biology. Springer, Cham, Switzerland, pp 53–60
Roosing S, Lamers IJ, de Vrieze E, van den Born LI, Lambertus S, Arts HH et al (2014) Disruption of the basal body protein POC1B results in autosomal-recessive cone-rod dystrophy. Am J Hum Genet 95:131–142. https://doi.org/10.1016/j.ajhg.2014.06.012
Durlu YK, Koroglu C, Tolun A (2014) Novel recessive cone-rod dystrophy caused by POC1B mutation. JAMA Ophthalmol 132:1185–1191. https://doi.org/10.1001/jamaophthalmol.2014.1658
Beck BB, Phillips JB, Bartram MP, Wegner J, Thoenes M, Pannes A et al (2014) Mutation of POC1B in a severe syndromic retinal ciliopathy. Hum Mutat 35:1153–1162. https://doi.org/10.1002/humu.22618
** X, Chen L, Wang D, Zhang Y, Chen Z, Huang H (2018) Novel compound heterozygous mutation in the POC1B gene underlie peripheral cone dystrophy in a Chinese family. Ophthalmic Genet 39:300–306. https://doi.org/10.1080/13816810.2018.1430239
Kominami A, Ueno S, Kominami T, Nakanishi A, Ito Y, Fu**ami K et al (2018) Case of cone dystrophy with normal fundus appearance associated with biallelic POC1B variants. Ophthalmic Genet 39:255–262. https://doi.org/10.1080/13816810.2017.1408846
Kameya S, Fu**ami K, Ueno S, Hayashi T, Kuniyoshi K, Ideta R et al (2019) Phenotypical characteristics of POC1B-associated retinopathy in Japanese cohort: cone dystrophy with normal funduscopic appearance. Invest Ophthalmol Vis Sci 60:3432–3446. https://doi.org/10.1167/iovs.19-26650
Peturson AC, Noel NCL, MacDonald IM (2021) A homozygous POC1B variant causes recessive cone-rod dystrophy. Ophthalmic Genet 42:349–353. https://doi.org/10.1080/13816810.2021.1894460
Robson AG, Frishman LJ, Grigg J, Hamilton R, Jeffrey BG, Kondo M et al (2022) ISCEV standard for full-field clinical electroretinography (2022 update). Doc Ophthalmol 144:165–177. https://doi.org/10.1007/s10633-022-09872-0
Ninomiya W, Mizobuchi K, Hayashi T, Okude S, Katagiri S, Kubo A et al (2020) Electroretinographic abnormalities associated with pregabalin: a case report. Doc Ophthalmol 140:279–287. https://doi.org/10.1007/s10633-019-09743-1
Sutter EE, Tran D (1992) The field topography of ERG components in man—I. The photopic luminance response. Vision Res 32:433–446. https://doi.org/10.1016/0042-6989(92)90235-b
Hayashi T, Tsuzuranuki S, Kozaki K, Urashima M, Tsuneoka H (2011) Macular dysfunction in Oguchi disease with the frequent mutation 1147delA in the SAG gene. Ophthalmic Res 46:175–180. https://doi.org/10.1159/000325024
Hayashi T, Mizobuchi K, Kameya S, Yoshitake K, Iwata T, Nakano T (2021) A new PDE6A missense variant p.Arg544Gln in rod-cone dystrophy. Doc Ophthalmol 143:107–114. https://doi.org/10.1007/s10633-021-09826-y
Hayashi T, Mizobuchi K, Kikuchi S, Nakano T (2021) Novel biallelic TRPM1 variants in an elderly patient with complete congenital stationary night blindness. Doc Ophthalmol 142:265–273. https://doi.org/10.1007/s10633-020-09798-5
Mizobuchi K, Hayashi T, Oishi N, Kubota D, Kameya S, Higasa K, et al (2021) Genotype-phenotype correlations in RP1-associated retinal dystrophies: A multi-center cohort study in Japan. J Clin Med 10. https://doi.org/10.3390/jcm10112265
Hayashi T, Murakami Y, Mizobuchi K, Koyanagi Y, Sonoda KH, Nakano T (2021) Complete congenital stationary night blindness associated with a novel NYX variant (p.Asn216Lys) in middle-aged and older adult patients. Ophthalmic Genet 42:412–419. https://doi.org/10.1080/13816810.2021.1904422
Mizobuchi K, Hayashi T, Yoshitake K, Fu**ami K, Tachibana T, Tsunoda K, et al (2020) Novel homozygous CLN3 missense variant in isolated retinal dystrophy: A case report and electron microscopic findings. Mol Genet Genomic Med 8:e1308. https://doi.org/10.1002/mgg3.1308
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–424. https://doi.org/10.1038/gim.2015.30
Tadaka S, Hishinuma E, Komaki S, Motoike IN, Kawashima J, Saigusa D et al (2021) jMorp updates in 2020: large enhancement of multi-omics data resources on the general Japanese population. Nucleic Acids Res 49:D536–D544. https://doi.org/10.1093/nar/gkaa1034
Weisschuh N, Mazzola P, Bertrand M, Haack TB, Wissinger B, Kohl S, et al (2021) Clinical characteristics of POC1B-associated retinopathy and assignment of pathogenicity to novel deep intronic and non-canonical splice site variants. Int J Mol Sci 22. https://doi.org/10.3390/ijms22105396
Ito N, Kameya S, Gocho K, Hayashi T, Kikuchi S, Katagiri S et al (2015) Multimodal imaging of a case of peripheral cone dystrophy. Doc Ophthalmol 130:241–251. https://doi.org/10.1007/s10633-015-9490-1
Akahori M, Tsunoda K, Miyake Y, Fukuda Y, Ishiura H, Tsuji S et al (2010) Dominant mutations in RP1L1 are responsible for occult macular dystrophy. Am J Hum Genet 87:424–429. https://doi.org/10.1016/j.ajhg.2010.08.009
Hayashi T, Gekka T, Kozaki K, Ohkuma Y, Tanaka I, Yamada H et al (2012) Autosomal dominant occult macular dystrophy with an RP1L1 mutation (R45W). Optom Vis Sci 89:684–691. https://doi.org/10.1097/OPX.0b013e31824eea32
Okuno T, Hayashi T, Sugasawa J, Oku H, Yamada H, Tsuneoka H et al (2013) Elderly case of pseudo-unilateral occult macular dystrophy with Arg45Trp mutation in RP1L1 gene. Doc Ophthalmol 127:141–146. https://doi.org/10.1007/s10633-013-9384-z
Fu**ami K, Kameya S, Kikuchi S, Ueno S, Kondo M, Hayashi T et al (2016) Novel RP1L1 variants and genotype-photoreceptor microstructural phenotype associations in Cohort of Japanese Patients with occult macular dystrophy. Invest Ophthalmol Vis Sci 57:4837–4846. https://doi.org/10.1167/iovs.16-19670
Davidson AE, Sergouniotis PI, Mackay DS, Wright GA, Waseem NH, Michaelides M et al (2013) RP1L1 variants are associated with a spectrum of inherited retinal diseases including retinitis pigmentosa and occult macular dystrophy. Hum Mutat 34:506–514. https://doi.org/10.1002/humu.22264
Birtel J, Eisenberger T, Gliem M, Muller PL, Herrmann P, Betz C et al (2018) Clinical and genetic characteristics of 251 consecutive patients with macular and cone/cone-rod dystrophy. Sci Rep 8:4824. https://doi.org/10.1038/s41598-018-22096-0
Acknowledgements
We would like to thank Ritsuko Nakayama for assistance with genetic analysis.
Funding
This work was supported, in part, by the Grants-in-Aid for Scientific Research (KAKENHI) Grant Number 21K09756 (TH) and research funds from Alcon (TH; Tokyo, Japan), Johnson and Johnson Vision, AMO (TH; Tokyo, Japan), Daiichi Sankyo (TH; Tokyo, Japan), Chugai (TH; Tokyo, Japan), Mitsubishi Tanabe Pharma (TH; Osaka, Japan), Senju (TH; Osaka, Japan), Bayer (TH; Osaka, Japan), Ritz medical (TH; Aichi, Japan), Uni-hite (TH; Kanagawa, Japan), and Kuribara (TH; Gunma, Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Takaaki Hayashi received research funds from Alcon (Tokyo, Japan), Johnson and Johnson Vision, AMO (Tokyo, Japan), Daiichi Sankyo (Tokyo, Japan), Chugai (Tokyo, Japan), Mitsubishi Tanabe Pharma (Osaka, Japan), Senju (Osaka, Japan), Bayer (Osaka, Japan), Ritz medical (Aichi, Japan), Uni-hite (Kanagawa, Japan), and Kuribara (Gunma, Japan). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of this manuscript.
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Statement on the welfare of animals
This article does not contain any studies with animals performed by any of the authors.
Informed consent
The patient provided informed consent for publishing this report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hayashi, T., Mizobuchi, K., Kameya, S. et al. A mild form of POC1B-associated retinal dystrophy with relatively preserved cone system function. Doc Ophthalmol 147, 59–70 (2023). https://doi.org/10.1007/s10633-023-09936-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-023-09936-9