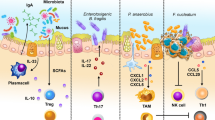
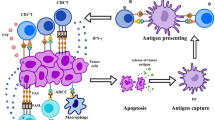
Abstract
Colorectal cancer (CRC) is one of the most frequent gastrointestinal malignant tumors worldwide. Immune checkpoint therapies (ICTs) have been proven to be a reliable treatment for some subtypes of CRC. Gut microbiome is closely involved in intestinal carcinogenesis through the regulation of local immune and inflammation of colonic mucosa. Numerous studies have demonstrated that the immunotherapeutic efficacy of CRC and other kinds of cancer is influenced by the immunosuppressive microenvironment constituted by intestinal microbiome and their metabolites. This Review will discuss the recent advances in how gut microbiome can modify the immune microenvironment and its potential role in ICTs of CRC.


Similar content being viewed by others
References
Siegel RL, Miller KD, Fuchs HE et al. Cancer statistics, 2021. CA Cancer J Clin 2021;71:7–33.
Feng Rui-Mei, Zong Yi-Nan, Cao Su-Mei et al. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond) 2019;29:22.
Perdue DG, Haverkamp D, Perkins C et al. Geographic variation in colorectal cancer incidence and mortality, age of onset, and stage at diagnosis among American Indian and Alaska Native people, 1990–2009. Am J Public Health 2014;104:S404–S414.
Giunta EF, Bregni G, Pretta A et al. Total neoadjuvant therapy for rectal cancer: making sense of the results from the RAPIDO and PRODIGE 23 trials. Cancer Treat Rev 2021;96:102177.
Yamazaki K, Nagase M, Tamagawa H et al. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann Oncol 2016;27:1539–1546.
Siegel RL, Miller KD, Fedewa SA et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177–193.
Lenz HJ, Van Cutsem E, Luisa Limon M et al. First-line Nivolumab Plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase II CheckMate 142 study. J Clin Oncol 2022;40:161–170.
Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321–330.
Patel J, Crawford JM. Microbiota-regulated outcomes of human cancer immunotherapy via the PD-1/PD-L1 axis. Biochemistry. 2018;57:901–903.
Montalban-Arques A, Katkeviciute E, Busenhart P et al. Commensal Clostridiales strains mediate effective anti-cancer immune response against solid tumors. Cell Host Microbe 2021;29:1573-1588.e7.
Yonekura S, Terrisse S, Alves Costa Silva C et al. Cancer induces a stress ileopathy depending on β-adrenergic receptors and promoting dysbiosis that contributes to carcinogenesis. Cancer Discov 2022;12:1128–1151.
Yang J, Wei H, Zhou Y et al. High-fat diet promotes colorectal tumorigenesis through modulating gut microbiota and metabolites. Gastroenterology 2022;162:135-149.e2.
Chen D, ** D, Huang S et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett 2020;469:456–467.
Gaines S, van Praagh JB, Williamson AJ et al. Western diet promotes intestinal colonization by collagenolytic microbes and promotes tumor formation after colorectal surgery. Gastroenterology 2020;158:958–970.
Partula V, Mondot S, Torres MJ et al. Associations between usual diet and gut microbiota composition: results from the Milieu Intérieur cross-sectional study. Am J Clin Nutr 2019;109:1472–1483.
Liu L, Tabung FK, Zhang X et al. Diets that promote colon inflammation associate with risk of colorectal carcinomas that contain Fusobacterium nucleatum. Clin Gastroenterol Hepatol 2018;16:1622-1631.e3.
Scott AJ, Alexander JL, Merrifield CA et al. International Cancer Microbiome Consortium consensus statement on the role of the human microbiome in carcinogenesis. Gut 2019;68:1624–1632.
Grosheva I, Zheng D, Levy M et al. High-throughput screen identifies host and microbiota regulators of intestinal barrier function. Gastroenterology 2020;S0016–5085:34921.
Oh NS, Lee JY, Kim YT et al. Cancer-protective effect of a synbiotic combination between Lactobacillus gasseri 505 and a Cudrania tricuspidata leaf extract on colitis-associated colorectal cancer. Gut Microbes 2020;12:1785803.
Farhana L, Nangia-Makker P, Arbit E et al. Bile acid: a potential inducer of colon cancer stem cells. Stem. Cell Res Ther 2016;1:181.
Perez-Lopez A, Behnsen J, Nuccio SP et al. Mucosal immunity to pathogenic intestinal bacteria. Nat Rev Immunol 2016;16:135–148.
Lozupone CA, Stombaugh JI, Gordon JI et al. Diversity, stability and resilience of the human gut microbiota. Nature 2012;489:220–230.
Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 2016;164:337–340.
Faith JJ, Guruge JL, Charbonneau M. The long-term stability of the human gut microbiota. Science 2013;341:1237439.
Yu J, Feng Q, Wong SH et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut 2017;66:70–78.
Lönnermark E, Nowrouzinan F, Adlerberth I et al. Oral and faecal lactobacilli and their expression of mannose-specific adhesins in individuals with and without IgA deficiency. Int J Med Microbiol 2012;302:53–60.
Sommer F, Backhed F. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol 2013;11:227–238.
Ozdal T, Sela DA, **ao J et al. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 2016;8:78.
Tao J, Li S, Gan RY, Zhao CN et al. Targeting gut microbiota with dietary components on cancer: effects and potential mechanisms of action. Crit Rev Food Sci Nutr 2020;60:1025–1037.
Gul L, Modos D, Fonseca S et al. Extracellular vesicles produced by the human commensal gut bacterium Bacteroides thetaiotaomicron affect host immune pathways in a cell-type specific manner that are altered in inflammatory bowel disease. J Extracell Vesicles 2022;11:e12189.
GomezdeAguero M, Ganal-Vonarburg SC, Fuhrer T et al. The maternal microbiota drives early postnatal innate immune development. Science 2016;351:1296–302.
Oh SF, Praveena T, Song H et al. Host immunomodulatory lipids created by symbionts from dietary amino acids. Nature 2021;600:302–307.
Poulsen AR, de Jonge N, Sugiharto S et al. The microbial community of the gut differs between piglets fed sow milk, milk replacer or bovine colostrum. Br J Nutr 2017;117:964–978.
Takiishi T, Fenero CIM, Câmara NOS. Intestinal barrier and gut microbiota: sha** our immune responses throughout life. Tissue Barriers 2017;5:e1373208.
Ge L, Qi J, Shao B et al. Microbial hydrogen economy alleviates colitis by reprogramming colonocyte metabolism and reinforcing intestinal barrier. Gut Microbes 2022;14:2013764.
Kim M, Qie Y, Park J, Kim CH. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 2016;20:202–214.
Furusawa Y, Obata Y, Fukuda S et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013;504:446–450.
Kang M, Martin A. Microbiome and colorectal cancer: unraveling host–microbiota interactions in colitis-associated colorectal cancer development. Semin Immunol 2017;32:3–13.
Aragon-Sanabria V, Kim GB, Dong C. From cancer immunoediting to new strategies in cancer immunotherapy: the roles of immune cells and mechanics in oncology. Adv Exp Med Biol 2018;1092:113–138.
Rutkowski MR, Stephen TL, Svoronos NA et al. Microbially driven TLR5-dependent signaling governs distal malignant progression through tumor-promoting inflammation. Cancer Cell 2015;27:27–40.
Gaudet RG, Zhu S, Halder A et al. A human apolipoprotein L with detergent-like activity kills intracellular pathogens. Science 2021;373:eabf8113.
Harusato A, Viennois E, Etienne-Mesmin L et al. Early-life microbiota exposure restricts myeloid-derived suppressor cell-driven colonic tumorigenesis. Cancer Immunol Res 2019;7:544–551.
Tamburini B, La Manna MP, La Barbera L et al. Immunity and nutrition: the right balance in inflammatory bowel disease. Cells 2022;11:455.
Purcell RV, Visnovska M, Biggs PJ et al. Distinct gut microbiome patterns associate with consensus molecular subtypes of colorectal cancer. Sci Rep 2017;7:11590.
Gur C, Ibrahim Y, Isaacson B et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015;42:344–355.
Wang X, Yang Y, Huycke MM. Commensal bacteria drive endogenous transformation and tumour stem cell marker expression through a bystander effect. Gut 2015;64:459–468.
Pohl JM, Gutweiler S, Thiebes S et al. Irf4-dependent CD103+CD11b+ dendritic cells and the intestinal microbiome regulate monocyte and macrophage activation and intestinal peristalsis in postoperative ileus. Gut 2018;67:379.
Lavoie S, Chun E, Bae S et al. Expression of free fatty acid receptor 2 by dendritic cells prevents their expression of interleukin 27 and is required for maintenance of mucosal barrier and immune response against colorectal tumors in mice. Gastroenterology 2020;158:1359–1372.
Saur IML, Panstruga R, Schulze-Lefert P. NOD-like receptor-mediated plant immunity: from structure to cell death. Nat Rev Immunol 2021;21:305–318.
Tye H, Yu CH, Simms LA et al. NLRP1 restricts butyrate producing commensals to exacerbate inflammatory bowel disease. Nat Commun 2018;9:3728.
Chen L, Wilson JE, Koenigsknecht MJ et al. NLRP12 attenuates colon inflammation by maintaining colonic microbial diversity and promoting protective commensal bacterial growth. Nat Immunol 2017;19:951.
Koblansky AA, Truax AD, Liu R et al. The innate immune receptor NLRX1 functions as a tumor suppressor by reducing colon tumorigenesis and key tumor-promoting signals. Cell Rep 2016;14:2562–2575.
Bekkering S, Domínguez-Andrés J, Joosten LAB, Riksen NP, Netea MG. Trained immunity: reprogramming innate immunity in health and disease. Annu Rev Immunol 2021;39:667–693.
Sorini C, Cardoso RF, Gagliani N, Villablanca EJ. Commensal bacteria-specific CD4+ T cell responses in health and disease. Front Immunol 2018;9:2667.
Wong SH, Zhao L, Zhang X et al. Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology 2017;153:1621-1633.e6.
Gu S, Zaidi S, Hassan MI et al. Mutated CEACAMs disrupt transforming growth factor beta signaling and alter the intestinal microbiome to promote colorectal carcinogenesis. Gastroenterology 2020;158:238–252.
Yu AI, Zhao L, Eaton KA et al. Gut microbiota modulate CD8 T cell responses to influence colitis-associated tumorigenesis. Cell Rep 2020;31:107471.
Kostic AD, Chun E, Robertson L et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013;14:207–215.
Mima K, Sukawa Y, Nishihara R et al. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol 2015;1:653–661.
Lopès A, Billard E, Casse AH et al. Colibactin-positive Escherichia coli induce a procarcinogenic immune environment leading to immunotherapy resistance in colorectal cancer. Int J Cancer 2020;146:3147–3159.
Thiele Orberg E, Fan H, Tam AJ et al. The myeloid immune signature of enterotoxigenic Bacteroides fragilis-induced murine colon tumorigenesis. Mucosal Immunol 2017;10:421–433.
Dejea CM, Fathi P, Craig JM et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 2018;359:592–597.
Kordahi MC, Stanaway IB, Avril M et al. Genomic and functional characterization of a mucosal symbiont involved in early-stage colorectal cancer. Cell Host Microbe 2021;29:1589-1598.e6.
Wang L, Tang L, Feng Y, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8+ T cells in mice. Gut, 2020; gutjnl-2019–320105.
Song X, Sun X, Oh SF et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 2020;577:410–415.
Hang S, Paik D, Yao L et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature 2020;579:E7.
Campbell C, McKenney PT, Konstantinovsky D et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 2020;581:475–479.
Yu R, Zuo F, Ma H, Chen S. Exopolysaccharide-producing Bifidobacterium adolescentis strains with similar adhesion property induce differential regulation of inflammatory immune response in Treg/Th17 axis of DSS-colitis mice. Nutrients 2019;11:782.
Vétizou M, Pitt JM, Daillère R et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015;350:1079–1084.
Sivan A, Corrales L, Hubert N et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015;350:1084–1089.
Chaput N, Lepage P, Coutzac C et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol 2019;30:2012.
Gopalakrishnan V, Spencer CN, Nezi L et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018;359:97–103.
Hakozaki T, Richard C, Elkrief A et al. The gut microbiome associates with immune checkpoint inhibition outcomes in patients with advanced non-small cell lung cancer. Cancer Immunol Res 2020;8:1243–1250.
Chaput N, Lepage P, Coutzac C et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol 2017;28:1368–1379.
Anderson R, Theron AJ, Rapoport BL. Immunopathogenesis of immune checkpoint inhibitor-related adverse events: roles of the intestinal microbiome and Th17 cells. Front Immunol 2019;10:2254.
Baruch EN, Youngster I, Ben-Betzalel G et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021;371:602–609.
Davar D, Dzutsev AK, McCulloch JA et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021;371:595–602.
Han K, Nam J, Xu J et al. Generation of systemic antitumour immunity via the in-situ modulation of the gut microbiome by an orally administered inulin gel. Nat Biomed Eng 2021;5:1377–1388.
Spencer CN, McQuade JL, Gopalakrishnan V et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 2021;374:1632–1640.
Buti S, Bersanelli M, Perrone F et al. Effect of concomitant medications with immune-modulatory properties on the outcomes of patients with advanced cancer treated with immune checkpoint inhibitors: development and validation of a novel prognostic index. Eur J Cancer 2021;142:18–28.
Pinato DJ, Howlett S, Ottaviani D et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer . JAMA Oncol 2019;5:1774–1778.
Tomita Y, Ikeda T, Sakata S et al. Association of probiotic Clostridium butyricum therapy with survival and response to immune checkpoint blockade in patients with lung cancer. Cancer Immunol Res 2020;8:1236–1242.
Routy B, Le Chatelier E, Derosa L et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018;359:91–97.
Matson V, Fessler J, Bao R et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018;359:104–108.
Wang F, Yin Q, Chen L et al. Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA-4 blockade. Proc Natl Acad Sci USA 2018;115:157–161.
Dubin K, Callahan MK, Ren B et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun 2016;7:10391.
Sun S, Luo L, Liang W et al. Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proc Natl Acad Sci USA 2020;117:27509–27515.
Daillère R, Vétizou M, Waldschmitt N et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 2016;45:931–943.
Griffin ME, Espinosa J, Becker JL et al. Enterococcus peptidoglycan remodeling promotes checkpoint inhibitor cancer immunotherapy. Science 2021;373:1040–1046.
Lee SH, Cho SY, Yoon Y et al. Bifidobacterium bifidum strains synergize with immune checkpoint inhibitors to reduce tumour burden in mice. Nat Microbiol 2021;6:277–288.
Mager LF, Burkhard R, Pett N et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 2020;369:1481–1489.
Lam KC, Araya RE, Huang A et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell 2021;184:5338-5356.e21.
Hezaveh K, Shinde RS, Klötgen A et al. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity 2022;55:324-340.e8.
Schluter J, Peled JU, Taylor BP et al. The gut microbiota is associated with immune cell dynamics in humans. Nature 2020;588:303–307.
Tanoue T, Morita S, Plichta DR et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 2019;565:600–605.
Roberti MP, Yonekura S, Duong CPM et al. Chemotherapy-induced ileal crypt apoptosis and the ileal microbiome shape immunosurveillance and prognosis of proximal colon cancer. Nat Med 2020;26:919–931.
Coutzac C, Jouniaux JM, Paci A et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat Commun 2020;11:2168.
Iida N, Dzutsev A, Stewart CA et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013;342:967–970.
Zheng JH, Nguyen VH, Jiang SN et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci Transl Med 2017;9:9537.
Peuker K, Strigli A, Tauriello DVF et al. Microbiota-dependent activation of the myeloid calcineurin-NFAT pathway inhibits B7H3- and B7H4-dependent anti-tumor immunity in colorectal cancer. Immunity 2022;55:701-717.e7.
Zhang SL, Mao YQ, Zhang ZY et al. Pectin supplement significantly enhanced the anti-PD-1 efficacy in tumor-bearing mice humanized with gut microbiota from patients with colorectal cancer. Theranostics 2021;11:4155–4170.
Huang AC, Orlowski RJ, Xu X et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med 2019;25:454–461.
Ferrara R, Mezquita L, Texier M et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol 2018;4:1543–1552.
Voorwerk L, Slagter M, Horlings HM et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med 2019;25:920–928.
Miao D, Margolis CA, Gao W et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science. 2018;359:801–806.
Pollack MH, Betof A, Dearden H et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol 2018;29:250–255.
Motzer RJ, Escudier B, McDermott DF et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J Immunother Cancer 2020;8:e000891.
Morad G, Helmink BA et al. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2022;3:576.
Goc J, Lv M, Bessman NJ et al. Dysregulation of ILC3s unleashes progression and immunotherapy resistance in colon cancer. Cell 2021;184:5015-5030.e16.
Le DT, Uram JN, Wang H et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–2520.
Chalabi M, Fanchi LF, Dijkstra KK et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med 2020;26:566–576.
Hamada T, Soong TR, Masugi Y et al. TIME (Tumor Immunity in the MicroEnvironment) classification based on tumor CD274 (PD-L1) expression status and tumor-infiltrating lymphocytes in colorectal carcinomas. Oncoimmunology 2018;7:e1442999.
Giannakis M, Mu XJ, Shukla SA et al. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep 2016;15:857–865.
Agaev A, Kotlov N, Nomie K et al. Conserved pan-cancer microenvironment subtypes predict response to immunotherapy. Cancer Cell 2021;39:845–865.
Shi L, Sheng J, Wang M et al. Combination therapy of TGF-β blockade and commensal-derived probiotics provides enhanced antitumor immune response and tumor suppression. Theranostics 2019;9:4115–4129.
Biza S, García-Cassani B, Ribeiro H et al. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature 2016;535:440–443.
Zhuo Q, Yu B, Zhou J et al. Lysates of Lactobacillus acidophilus combined with CTLA-4-blocking antibodies enhance antitumor immunity in a mouse colon cancer model. Sci Rep 2019;9:20128.
Ji SK, Yan H, Jiang T et al. Preparing the gut with antibiotics enhances gut microbiota reprogramming efficiency by promoting xenomicrobiota colonization. Front Microbiol 2017;8:1208.
Costello SP, Hughes PA, Waters O et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA 2019;321:156–164.
Acknowledgments
This study was supported by the Xuzhou Medical key research and development project (2018103011), General project of Jiangsu Health Committee (H2019047).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, L., Xu, Y. & Feng, M. Role of Gut Microbiome in Immune Regulation and Immune Checkpoint Therapy of Colorectal Cancer. Dig Dis Sci 68, 370–379 (2023). https://doi.org/10.1007/s10620-022-07689-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-022-07689-0