Abstract
Background and Aim
FAST score has a good performance for diagnosing the composite of NASH + NAS ≥ 4 + F ≥ 2. However, it has not been evaluated in Latin American individuals with nonalcoholic fatty liver disease (NAFLD). We aimed to analyze the performance of the FAST score in a Brazilian NAFLD population.
Methods
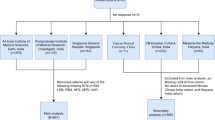
Cross-sectional study was held in ≥ 18 years NAFLD patients diagnosed by ultrasonography and submitted to liver biopsy (LB). Liver stiffness (LSM) and CAP measurements were performed with FibroScan®, using M (BMI < 32 kg/m2) or XL probes. Area under receiver operating characteristic (AUROC) curves were calculated as well as sensitivity (S), specificity (Spe), positive predictive value (VPP) and negative predictive value (NPV) for the previously established FAST score cut-offs.
Results
Among 287 patients included (75% female; mean age 55 ± 10 years), NASH + NAS ≥ 4 + F ≥ 2 was reported in 30% of LB. For the FAST cut-off of 0.35, the S and NPV to rule out NASH + NAS ≥ 4 + F ≥ 2 were 78.8% and 87.8%, respectively. Regarding the cut-off of 0.67, the Spe and PPV to rule-in NASH + NAS ≥ 4 + F ≥ 2 were 89.1%, 61.8%, respectively. The AUROC of FAST for all included patients was 0.78 (95% CI 0.72–0.84) and for those with ≥ 32 kg/m2 was 0.81 (95% CI 0.74–0.88).
Conclusion
FAST score has a good performance in a Brazilian NAFLD population, even in patients with higher BMI when the XL probe is adopted. Therefore, FAST can be used as a noninvasive screening tool mainly for excluding the diagnosis of progressive NASH, reducing the number of unnecessary liver biopsies.



Similar content being viewed by others
Abbreviations
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Steatohepatitis
- TE:
-
Transient elastography
- CAP:
-
Controlled Attenuation Parameter
- LSM:
-
Liver stiffness measurement
- AST:
-
Aspartate aminotransferase
- FAST score:
-
FibroScan®-AST
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
- HIV:
-
Human immunodeficiency virus
- HCV:
-
Hepatitis C virus
- HBV:
-
Hepatitis B virus
- ICF:
-
Informed consent form
- BMI:
-
Body mass index
- ALT:
-
Alanine aminotransferase
- GGT:
-
Gammaglutamil transferase
- kPa:
-
Kilopascal
- dB/m:
-
Decibel/meter
- OR:
-
Odds ratios
- AUROC:
-
Area under receiver operating characteristic
- T2DM:
-
Type 2 diabetes
- SAH:
-
Systemic arterial hypertension
- S:
-
Sensitivity
- Spe:
-
Specificity
References
Williams CD, Stengel J, Asike MI et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131.
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84.
Kleiner DE, Brunt EM, Natta MV et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321.
Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20:475–485. https://doi.org/10.3748/wjg.v20.i2.475.
Neuberger J, Patel J, Caldwell H et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut. 2020;69:1382–1403. https://doi.org/10.1136/gutjnl-2020-321299.
Ekstedt M, Hagstrom H, Nasr P et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554.
Sanyal AJ. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 2019;16:377–386.
Younossi ZM, Loomba R, Rinella ME et al. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2018;68:361–371. https://doi.org/10.1002/hep.29724.
Cardoso AC, de Figueiredo-Mendes C, Villela-Nogueira CA, Sanyal AJ. New drugs for non-alcoholic steatohepatitis. Liver Int. 2020;40:96–101. https://doi.org/10.1111/liv.14354.
Wong GL, Wong VW-S, Choi PC-L et al. Assessment of fibrosis by transient elastography compared with liver biopsy and morphometry in chronic liver diseases. Clin Gastroenterol Hepatol 2008;6:1027–35.
Yoneda M, Yoneda M, Mawatari H et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD). Dig Liver Dis 2008;40:371–378.
Wong VW, Vergniol J, Wong GL-H et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010;51:454–462.
Bellentani S, Saccoccio G, Masutti F et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med 2000;132:112–117.
Castera L, Foucher J, Bernard P-H et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 2010;51:828–835.
Wong GL-H, Wong VW-S, Chim AM-L et al. Factors associated with unreliable liver stiffness measurement and its failure with transient elastography in the Chinese population. J Gastroenterol Hepatol 2011;26:300–5.
de Lédinghen V, Vergniol J, Foucher J, El-Hajbi F, Merrouche W, Rigalleau V. Feasibility of liver transient elastography with FibroScan using a new probe for obese patients. Liver Int. 2010;30:1043–1048.
Friedrich-Rust M, Hadji-Hosseini H, Kriener S et al. Transient elastography with a new probe for obese patients for non-invasive staging of non-alcoholic steatohepatitis. Eur Radiol. 2010;20:2390–2396.
Wong VW-S, Vergniol J, Wong GL-H et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2012;107:1862–71.
Sasso M, Beaugrand M, de Ledinghen V et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–1835.
de Lédinghen V, Vergniol J, Foucher J, Merrouche W, le Bail B. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012;32:911–918.
de Lédinghen V, Wong VW-S, Vergniol J et al. Diagnosis of liver fibrosis and cirrhosis using liver stiffness measurement: comparison between M and XL probe of FibroScan. J Hepatol 2012;56:833–9.
de Lédinghen V, Hiriart JB, Vergniol J, Merrouche W, Bedossa P, Paradis V. Controlled attenuation parameter (CAP) with the XL probe of the Fibroscan®: a comparative study with the M probe and liver biopsy. Dig Dis Sci 2017;62:2569–2577. https://doi.org/10.1007/s10620-017-4638-3.
Cardoso AC, Cravo C, Calçado FL et al. The performance of M and XL probes of FibroScan for the diagnosis of steatosis and fibrosis on a Brazilian nonalcoholic fatty liver disease cohort. Eur J Gastroentrol Hepatol. 2020;32:231–238. https://doi.org/10.1097/MEG.0000000000001496.
Newsome PN, Sasso M, Deeks JJ et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:362–373. https://doi.org/10.1016/S2468-1253(19)30383-8.
Sandrin L, Fourquet B, Hasquenoph J-M et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 2003;29:1705–1713.
de Lédinghen V, Vergniol J. Transient elastography (FibroScan). Gastroenterol Clin Biol. 2008;32:58–67.
Idalsoaga F, Kulkarni AV, Mousa OY, Arrese M, Arab JP. Non-alcoholic fatty liver disease and alcohol-related liver disease: two intertwined entities. Front Med (Lausanne) 2020;7:448. https://doi.org/10.3389/fmed.2020.00448.
Weston SR, Leyden W, Murphy R et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology 2005;41:372–379. https://doi.org/10.1002/hep.20554.
Parra FC, Amado RC, Lambertucci JR, Rocha J, Antunes CM, Pena SDJ. Color and genomic ancestry in Brazilians. Proc Natl Acad Sci USA 2003;100:177–182. https://doi.org/10.1073/pnas.0126614100.
Alves-Silva J, Da Silva Santos M, Guimarães PE. The ancestry of Brazilian mtDNA lineages. Am J Hum Genet. 2000;67:444–461. https://doi.org/10.1086/303004.
Cotrim HP, Parise ER, Oliveira CPMS et al. Nonalcoholic fatty liver disease in Brazil. Clinical and histological profile. Ann Hepatol. 2011;10:33–37.
Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. 2020;158:1851–1864. https://doi.org/10.1053/j.gastro.2020.01.052.
Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:511–531. https://doi.org/10.1016/j.cld.2009.07.005.
Oeda S, Takahashi H, Imajo K et al. Diagnostic accuracy of FibroScan-AST score to identify non-alcoholic steatohepatitis with significant activity and fibrosis in Japanese patients with non-alcoholic fatty liver disease: comparison between M and XL probes. Hepatol Res. 2020;50:831–839. https://doi.org/10.1111/hepr.13508.
Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1264-1281.e4. https://doi.org/10.1053/j.gastro.2018.12.036.
Funding
This work was supported by funding from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Rio de Janeiro, and Conselho Nacional de Desenvolvimento Científico e Tecnológico, (CNPq), Brazil.
Author information
Authors and Affiliations
Contributions
Cristiane A. Villela-Nogueira and Ana Carolina Cardoso contributed to the study conception and design. Material preparation and data collection were performed by Ana Carolina Cardoso, Cristiane Valle Tovo, Nathalie Carvalho Leite, Ibrahim A El Bacha, Fernanda Luiza Calçado, Gabriela Perdomo Coral, Glauco Navas Sammarco, Claudia Cravo, Roberto José Carvalho Filho, Renata de Mello Perez, Edison Roberto Parise and Cristiane A. Villela-Nogueira. Statistical analysis was performed by Ronir Raggio Luiz and Cristiane A. Villela-Nogueira. The first draft of the manuscript was written by Ana Carolina Cardoso, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have nothing to disclose.
Ethics approval
All procedures performed in this study were following the ethical standards of the institutional and national research committee and with the 1964 Declaration of Helsinki and its later amendments. The local Ethics Committee (Hospital Universitário Clementino Fraga Filho) approved the study (CAAE No. 38752414.7.0000.5257).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cardoso, A.C., Tovo, C.V., Leite, N.C. et al. Validation and Performance of FibroScan®-AST (FAST) Score on a Brazilian Population with Nonalcoholic Fatty Liver Disease. Dig Dis Sci 67, 5272–5279 (2022). https://doi.org/10.1007/s10620-021-07363-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-07363-x