Abstract
Background
Alcoholic hepatitis (AH) is a serious clinical syndrome often associated with muscle wasting. Myostatin, a member of the transforming growth factor-β superfamily, has been studied in diseases with muscle wasting; however, the role of myostatin in AH is unknown.
Aims
To investigate the association between myostatin, clinical variables, and outcomes in AH.
Methods
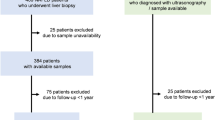
We analyzed data for cases of AH and controls of heavy drinkers (HD) in TREAT001 (NCT02172898) with serum myostatin levels (AH: n = 131, HD: n = 124). We compared characteristics between the two groups at baseline, 30, and 90 days and explored correlations between myostatin and clinical variables. We then modeled the relationship of myostatin to other variables, including mortality.
Results
Baseline median myostatin was lower in AH compared to HD (males: 1.58 vs 3.06 ng/ml, p < 0.001; females: 0.84 vs 2.01 ng/ml, p < 0.001). In multivariable linear regression, bilirubin, WBC, and platelet count remained negatively correlated with myostatin in AH. AH females who died at 90 days had significantly lower myostatin, but in a multivariable logistic model with MELD and myostatin, only MELD remained significantly associated with 90-day mortality. During 1-year follow-up, AH cases (n = 30) demonstrated an increase in myostatin (mean, 1.73 ng/ml) which correlated with decreasing MELD scores (ρ = − 0.42, p = 0.01).
Conclusions
Myostatin levels are significantly lower in AH compared to HD and are negatively correlated with total bilirubin, WBC, and platelet count. Myostatin increased as patients experienced decreases in MELD. Overall, myostatin demonstrated a dynamic relationship with AH outcomes and future studies are needed to understand the prognostic role of myostatin in AH.





Similar content being viewed by others
Abbreviations
- AH:
-
Alcoholic hepatitis
- HD:
-
Heavy drinking
- TREAT001:
-
Translational Research and Evolving Alcoholic Hepatitis Treatment 001 Study
- INR:
-
International normalized ratio
- WBC:
-
White blood cells
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- ALP:
-
Alkaline phosphatase
- MELD:
-
Model for End-Stage Liver Disease
- DF:
-
Discriminate factor
- TGF-®:
-
Transforming growth factor-®
- GDF-8:
-
Growth differentiation factor-8
- ELISA:
-
Enzyme-linked immunosorbent assay
- AIC:
-
Akaike information criterion
References
Mellinger JL. Epidemiology of alcohol use and alcoholic liver disease. Clin Liver Dis. 2019;13:136–139.
Dang K, Hirode G, Singal AK, Sundaram V, Wong RJ. Alcoholic liver disease epidemiology in the United States: a retrospective analysis of 3 US databases. Am J Gastroenterol. 2020;115:96–104.
White AM, Castle I-JP, Hingson RW, Powell PA. Using death certificates to explore changes in alcohol-related mortality in the United States, 1999 to 2017. Alcohol Clin Exp Res. 2020;44:178–187.
Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and treatment of alcohol-associated liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71:306–333.
Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–2769.
Gholam PM. Prognosis and prognostic scoring models for alcoholic liver disease and acute alcoholic hepatitis. Clin Liver Dis. 2016;20:491–497.
Bennett K, Enki DG, Thursz M, Cramp ME, Dhanda AD. Systematic review with meta-analysis: high mortality in patients with non-severe alcoholic hepatitis. Aliment Pharmacol Ther. 2019;50:249–257.
Hughes E, Hopkins LJ, Parker R. Survival from alcoholic hepatitis has not improved over time. PloS One. 2018;13:e0192393.
Chayanupatkul M. Alcoholic hepatitis: a comprehensive review of pathogenesis and treatment. World J Gastroenterol. 2014;20:6279.
Thursz M, Kamath PS, Mathurin P, Szabo G, Shah VH. Alcohol-related liver disease: areas of consensus, unmet needs and opportunities for further study. J Hepatol. 2019;70:521–530.
McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90.
Zimmers TA, Davies MV, Koniaris LG, et al. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296:1486–1488.
Peng L-N, Lee W-J, Liu L-K, Lin M-H, Chen L-K. Healthy community-living older men differ from women in associations between myostatin levels and skeletal muscle mass: myostatin levels and skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2018;9:635–642.
Fife E, Kostka J, Kroc Ł, et al. Relationship of muscle function to circulating myostatin, follistatin and GDF11 in older women and men. BMC Geriatr. 2018;18:200.
Furihata T, Kinugawa S, Fukushima A, et al. Serum myostatin levels are independently associated with skeletal muscle wasting in patients with heart failure. Int J Cardiol. 2016;220:483–487.
Ju C-R, Chen R-C. Serum myostatin levels and skeletal muscle wasting in chronic obstructive pulmonary disease. Respir Med. 2012;106:102–108.
Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31.
Mauro E, Crespo G, Martinez-Garmendia A, et al. Cystatin C and sarcopenia predict acute on chronic liver failure development and mortality in patients on the liver transplant waiting list. Transplantation. 2020;104:e188–e198.
Vural A, Attaway A, Welch N, Zein J, Dasarathy S. Skeletal muscle loss phenotype in cirrhosis: a nationwide analysis of hospitalized patients. Clin Nutr. 2020. https://doi.org/10.1016/j.clnu.2020.03.032.
García PS, Cabbabe A, Kambadur R, Nicholas G, Csete M. Elevated myostatin levels in patients with liver disease: a potential contributor to skeletal muscle wasting. Anesth Analg. 2010;111:707–709.
Nishikawa H, Enomoto H, Ishii A, et al. Elevated serum myostatin level is associated with worse survival in patients with liver cirrhosis: myostatin and liver cirrhosis. J Cachexia Sarcopenia Muscle. 2017;8:915–925.
Skladany L, Koller T, Molcan P, et al. Prognostic usefulness of serum myostatin in advanced chronic liver disease: its relation to gender and correlation with inflammatory status. J Physiol Pharmacol Off J Pol Physiol Soc. 2019;. https://doi.org/10.26402/jpp.2019.3.03.
Ebadi M, Bhanji RA, Mazurak VC, Montano-Loza AJ. Sarcopenia in cirrhosis: from pathogenesis to interventions. J Gastroenterol. 2019;54:845–859.
Welch N, Dasarathy J, Runkana A, et al. Continued muscle loss increases mortality in cirrhosis: impact of aetiology of liver disease. Liver Int. 2020;40:1178–1188.
Al-Azzawi Y, Albo B, Fasullo M, et al. Sarcopenia is associated with longer hospital stay and multiorgan dysfunction in alcoholic hepatitis. Eur J Gastroenterol Hepatol. 2020;32:733–738.
Lang CH, Frost RA, Svanberg E, Vary TC. IGF-I/IGFBP-3 ameliorates alterations in protein synthesis, eIF4E availability, and myostatin in alcohol-fed rats. Am J Physiol Endocrinol Metab. 2004;286:E916–E926.
Liangpunsakul S, Puri P, Shah VH, et al. Effects of age, sex, body weight, and quantity of alcohol consumption on occurrence and severity of alcoholic hepatitis. Clin Gastroenterol Hepatol. 2016;14:1831–1838.e3.
Liangpunsakul S, Beaudoin JJ, Shah VH, et al. Interaction between the patatin-like phospholipase domain-containing protein 3 genotype and coffee drinking and the risk for acute alcoholic hepatitis. Hepatol Commun. 2018;2:29–34.
RStudio Team. RStudio: integrated development environment for R. Boston, MA: RStudio, Inc.; 2018.
Wickham H. ggplot2: Elegant graphics for data analysis. Berlin: Springer; 2016.
Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009;9:447–453.
Li Y, Zhang F, Modrak S, Little A, Zhang H. Chronic alcohol consumption enhances skeletal muscle wasting in mice bearing cachectic cancers: the role of TNFα/myostatin axis. Alcohol Clin Exp Res. 2020;44:66–77.
Yano S, Nagai A, Isomura M, et al. Relationship between blood myostatin levels and kidney function: shimane cohre study. PloS One. 2015;10:e0141035.
Crabb DW, Bataller R, Chalasani NP, et al. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA alcoholic hepatitis consortia. Gastroenterology. 2016;150:785–790.
Funding
APD is funded by the American Association for the Study of Liver Disease Foundation’s 2017 Career Development Award. Collection of serum samples and serum myostatin analysis was funded by the NIH 3U01AA021840-07S1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
For full disclosure, Dr. Naga Chalasani has ongoing paid consulting activities (or had in preceding 12 months) with NuSirt, Abbvie, Afimmune (DS Biopharma), Allergan (Tobira), Madrigal, Siemens, Foresite, Galectin, Zydus, and La Jolla. These consulting activities are generally in the areas of nonalcoholic fatty liver disease and drug hepatotoxicity. Dr. Chalasani receives research grant support from Exact Sciences, Intercept, and Galectin Therapeutics where his institution receives the funding. Over the last decade, Dr. Chalasani has served as a paid consultant to more than 35 pharmaceutical companies and these outside activities have regularly been disclosed to his institutional authorities.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shamseddeen, H., Madathanapalli, A., Are, V.S. et al. Changes in Serum Myostatin Levels in Alcoholic Hepatitis Correlate with Improvement in MELD. Dig Dis Sci 66, 3062–3073 (2021). https://doi.org/10.1007/s10620-020-06632-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06632-5