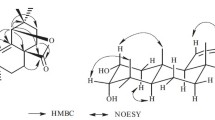
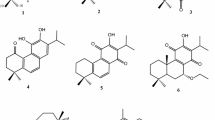
Phytochemical investigation of the leaves of Guarea convergens T. D. Penn. led to the isolation of new apotirucallane triterpenes 24-acetoxy-25-hydroxy-3,7-dioxoapotirucalla-14-en-21,23-olide and 7α,24,25-trihydroxy-3-oxoapotirucalla-14-en-21,23-olide, steroid ergosta-5,24(24′)-diene-3β,7α,21-triol, in addition to the known ergosta-5,24(24′)-diene-3β,4β,22S-triol. The investigation from branches yielded the tirucallane triterpenes melianone and melianodiol. This is the first chemical study of this species, whose identified molecules contribute to knowledge of the biodiversity of the Amazonian forestan for chemosystematics of the Rutales order.
Similar content being viewed by others
References
V. V. Grishko, I. A. Tolmacheva, and A. V. Pereslavtseva, Chem. Nat. Compd., 51, 1 (2015).
J. H. G. Lago, C. B. Brochini, and N. F. Roque, Phytochemistry, 55, 727 (2000).
J. H. G. Lago and N. F. Roque, Phytochemistry, 60, 329 (2002).
M. Furlan, N. F. Roque, and W. Wolter-Filho, Phytochemistry, 32, 1519 (1993).
J. H. G. Lago, C. B. Brochini, and N. F. Roque, Phytochemistry, 60, 333 (2002a).
A. Jimenez, C. Villarreal, R. A. Toscano, M. Cook, J. T. Arnason, R. Bye, and R. Mata, Phytochemistry, 49, 1981 (1998).
C. H. Miquita, C. S. Barbosa, L. Hamerski, U. C. Sarmento, J. N. Nascimento, W. S. Garcez, and F. R. Garcez, Molecules, 20, 111 (2015).
A. P. Lins, M. M. Braggio, J. D. Felicio, A. M. Giuriatti, and J. C. Felicio, Rev. Latinoamer. Quim., 23, 30 (1992).
G. Ferguson, P. A. Gunn, W. C. Marsh, R. McCrindle, R. Restivo, J. D. Connolly, J. W. B. Fulke, and M. S. Henderson, J. Chem. Soc. Chem. Commun., 5, 159 (1973).
O. L. Pereira-Junior, W. Wolter-Filho, A. F. I. Rocha, M. G. Carvalho, and R. Braz-Filho, Quim. Nova, 13, 247 (1990).
W. W. Harding, H. Jacobs, S. Mclean, and W. F. Reynolds, Magn. Reson. Chem., 39, 719 (2001).
M. R. Camacho; J. D. Phillipson, S. L. Croft, G. C. Kirby, D. C. Warhurst, and P. N. Solis, Phytochemistry, 56, 203 (2001).
D. A. Mulholland, T. V. Monkhe, D. A. H. Taylor, and M. S. Sajab, Phytochemistry, 52, 123 (1999).
A. F. Barrero, J. F. Sanchez, E. J. Alvarez-Manzaneda, M. M. Dorado, and A. Haidour, Phytochemistry, 32, 1261 (1993).
Y. Letourneux, Q. Khuong-Huu, M. Gut, and G. Lukacs, J. Org. Chem., 40, 1674 (1975).
W. W. Harding, H. Jacobs, P. A. Lewis, S. McLean, and W. F. Reynolds, Nat. Prod. Lett., 15, 253 (2001).
U. S. Cunha, J. D. Vendramim, W. C. Rocha, and P. C. Vieira, Neotrop. Entomol., 37, 709 (2008).
A. Guerriero, M. D′ambrosio, and F. J. Pietra, J. Nat. Prod., 56, 1962 (1993).
V. U. Ahmad, R. Aliya, S. Perveen, and M. Shameel, Phytochemistry, 33, 1189 (1993).
M. V. D′Auria, E. Finamore, L. Minale, C. Pizza, R. Riccio, F. Zollo, M. Pusset, and P. Tirard, J. Chem. Soc. Perkin Trans. 1, 2277 (1984).
T. Nakashi, A. Inada, and D. Lavie, Chem. Pharm. Bull., 34, 100 (1986).
N. G. Ntalli, F. Cottiglia, C. A. Bueno, L. E. Alche, M. Leonti, S. Vargiu, E. Bifulco, U. Menkissoglu-Spiroudi, and P. Caboni, Molecules, 15, 5866 (2010).
Acknowledgment
The authors are grateful to the Fundacao de Amparo a Pesquisa do Estado do Amazonas (FAPEAM) for financial support, and to the Coordenacao de Aperfeicoamento de Pessoal de Nivel (CAPES) for the Master′s scholarship awarded to Willian Hayasida.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 2, March–April, 2017, pp. 265–269.
Rights and permissions
About this article
Cite this article
Hayasida, W., Oliveira, L.M., Ferreira, A.G. et al. Ergostane Steroids, Tirucallane and Apotirucallane Triterpenes from Guarea convergens . Chem Nat Compd 53, 312–317 (2017). https://doi.org/10.1007/s10600-017-1977-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-017-1977-4