Abstract
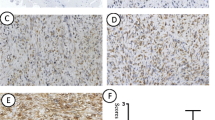
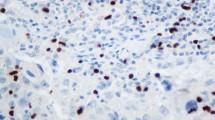
Hox genes encode a family of homeodomain-containing transcription factors that determine cellular identity during development and which are also expressed in some types of cancer. The HOXD3 gene, a member of the Hox gene family, has been demonstrated to be expressed in several tumor cell lines, which exhibit enhanced invasion and metastasis through coordinate expression of metastasis-associated factors. However, the clinical impact of HOXD3 in breast cancer remains unclear. In the current study, we examined the expression of HOXD3 and integrin β3 by immunohistochemical staining in patients with invasive breast cancer. We found that HOXD3 expression was significantly frequent in high histopathological grade and hormone-receptor negative breast cancer patients. The expression of HOXD3 was closely associated with integrin β3 expression. Furthermore, patients with high HOXD3 expression levels in their breast tumors had significantly shorter survival times than patients in which HOXD3 was weakly expressed in breast tumors. Univariate and multivariate analyses confirmed that increased HOXD3-expression was an independent and significant factor in predicting poor prognosis for patients with breast cancer. In conclusion, HOXD3 expression is a significant unfavorable prognostic factor for patients with invasive breast cancer and as such is a potentially useful prognostic marker for breast cancer.




Similar content being viewed by others
Abbreviations
- MMP:
-
Matrix metalloproteinase
- WHO:
-
World Health Organization
- DFS:
-
Disease-free survival
- OS:
-
Overall survival
References
Gehring WJ, Hiromi Y (1986) Homeotic genes and the homeobox. Annu Rev Genet 20:147–173
Scott MP (1992) Vertebrate homeobox gene nomenclature. Cell 71:551–553
Graham A, Papalopulu N, Krumlauf R (1989) The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell 57:367–378
McGinnis W, Krumlauf R (1992) Homeobox genes and axial patterning. Cell 68:283–302
Levine M, Hoey T (1988) Homeobox proteins as sequence-specific transcription factors. Cell 55:537–540
Ruddle FH et al (1994) Evolution of Hox genes. Annu Rev Genet 28:423–442
Mark M, Rijli FM, Chambon P (1997) Homeobox genes in embryogenesis and pathogenesis. Pediatr Res 42:421–429
Takahashi Y et al (2004) Expression profiles of 39 HOX genes in normal human adult organs and anaplastic thyroid cancer cell lines by quantitative real-time RT-PCR system. Exp Cell Res 293:144–153
Abate-Shen C (2002) Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer 2:777–785
Raman V et al (2000) Compromised HOXA5 function can limit p53 expression in human breast tumours. Nature 405:974–978
Trivedi CM, Patel RC, Patel CV (2007) Homeobox gene HOXA9 inhibits nuclear factor-kappa B dependent activation of endothelium. Atherosclerosis 195:e50–e60
Trivedi CM, Patel RC, Patel CV (2008) Differential regulation of HOXA9 expression by nuclear factor kappa B (NF-kappaB) and HOXA9. Gene 408:187–195
Wu X et al (2006) HOXB7, a homeodomain protein, is overexpressed in breast cancer and confers epithelial–mesenchymal transition. Cancer Res 66:9527–9534
Condie BG, Capecchi MR (1993) Mice homozygous for a targeted disruption of Hoxd-3 (Hox-4.1) exhibit anterior transformations of the first and second cervical vertebrae, the atlas and the axis. Development 119:579–595
Manley NR, Capecchi MR (1998) Hox group 3 paralogs regulate the development and migration of the thymus, thyroid, and parathyroid glands. Dev Biol 195:1–15
Hamada J et al (2001) Overexpression of homeobox gene HOXD3 induces coordinate expression of metastasis-related genes in human lung cancer cells. Int J Cancer 93:516–525
Taniguchi Y, Komatsu N, Moriuchi T (1995) Overexpression of the HOX4A (HOXD3) homeobox gene in human erythroleukemia HEL cells results in altered adhesive properties. Blood 85:2786–2794
Haybittle JL et al (1982) A prognostic index in primary breast cancer. Br J Cancer 45:361–366
Kowalski PJ, Rubin MA, Kleer CG (2003) E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res 5:R217–R222
Huber GF et al (2011) Down regulation of E-cadherin (ECAD)—a predictor for occult metastatic disease in sentinel node biopsy of early squamous cell carcinomas of the oral cavity and oropharynx. BMC cancer 11(217):1–8
Kashiwagi S et al (2010) Significance of E-cadherin expression in triple-negative breast cancer. Br J Cancer 103:249–255
Frixen UH et al (1991) E-cadherin-mediated cell–cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol 113:173–185
Vleminckx K et al (1991) Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 66:107–119
Watabe M et al (1994) Induction of polarized cell–cell association and retardation of growth by activation of the E-cadherin-catenin adhesion system in a dispersed carcinoma line. J Cell Biol 127:247–256
Mariotti A et al (2007) N-cadherin as a therapeutic target in cancer. Expert Opin Investig Drugs 16:451–465
Cavallaro U (2004) N-cadherin as an invasion promoter: a novel target for antitumor therapy? Curr Opin Investig Drugs 5:1274–1278
Hazan RB et al (2000) Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol 148:779–790
Planaguma J et al (2011) Matrix metalloproteinase-2 and matrix metalloproteinase-9 codistribute with transcription factors RUNX1/AML1 and ETV5/ERM at the invasive front of endometrial and ovarian carcinoma. Hum Pathol 42:57–67
Shen Q et al (2010) Tissue inhibitor of metalloproteinase-2 regulates matrix metalloproteinase-2-mediated endothelial barrier dysfunction and breast cancer cell transmigration through lung microvascular endothelial cells. Mol Cancer Res 8:939–951
Takayama S et al (2005) The relationship between bone metastasis from human breast cancer and integrin alpha(v)beta3 expression. Anticancer Res 25:79–83
Scatena M et al (1998) NF-kappaB mediates alphavbeta3 integrin-induced endothelial cell survival. J Cell Biol 141:1083–1093
Matter ML, Ruoslahti E (2001) A signaling pathway from the alpha5beta1 and alpha(v)beta3 integrins that elevates bcl-2 transcription. J Biol Chem 276:27757–27763
De S et al (2005) VEGF-integrin interplay controls tumor growth and vascularization. Proc Natl Acad Sci USA 102:7589–7594
Acknowledgments
The study was supported by Nature Science Foundation of Heilongjiang Province (C160403).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Cheng Shaoqiang and Zhang Yue are contributed equally to this study and should be considered co-first authors.
Rights and permissions
About this article
Cite this article
Shaoqiang, C., Yue, Z., Yang, L. et al. Expression of HOXD3 correlates with shorter survival in patients with invasive breast cancer. Clin Exp Metastasis 30, 155–163 (2013). https://doi.org/10.1007/s10585-012-9524-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-012-9524-y