Abstract
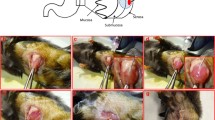
We describe the generation of two orthotopic murine models for endometrial cancer (EC).The first model is generated from endometrial Hec-1A cancer cells transfected with luciferase and injected directly into the uterus of female mice. This model allows a follow-up with bioluminescence imaging (BLI) along the experiment and generates abdominal dissemination and lymphatic and hematogenous metastases in high percentages, also detectables with BLI. The dissemination pattern of this model imitates the advanced stages of EC in patients, and its molecular profile corresponds to aggressive type 2 EC (p53 positive, hormone receptors negative, high percentage of Ki67 positive cells). The second model is derived from endometrioid human tissue collected from surgical pieces. By injecting this tissue inside the uterine cavity of a mouse we obtain orthotopic growth with pelvic dissemination and lymph node metastasis. The molecular pattern observed in human type 1 endometrioid EC (p53 negative, low Ki67 index, presence of hormone receptors) is conserved after the murine growth in orthotopic tumor and metastases. This model supposes a singular pre-clinical tool to study therapeutic agents, though it mimics clinical and molecular behavior of endometrioid EC, which is the most common histology in the patient.



Similar content being viewed by others
Abbreviations
- EC:
-
Endometrial cancer
- BLI:
-
Bioluminescence imaging
- Hec1A:
-
Human endometrial carcinoma 1A
- Fluc:
-
Firefly luciferase
- Hec1A-Fluc:
-
Hec1A cells transfected with luciferase gen
- FIGO:
-
International Federation of Gynecology and Obstetrics
- i.p:
-
Intraperitoneal
- ROI:
-
Region of interest
- ph/s:
-
Photons/second
- H&E:
-
Hematoxylin and eosin
- IHC:
-
Immunohistochemical
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- MSH6:
-
MutS homolog 6 gene
- MSH2:
-
MutS homolog 2 gene
- MLH1:
-
MutL homolog 1 gene
- SEM:
-
Standard errors of the mean
- 3D:
-
Three-dimensional
References
Baekelandt MM, Castiglione M (2009) ESMO Guidelines Working group.Endometrial carcinoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 20(4):iv29–iv31
Homesley HD, Filiaci V, Gibbons SK et al (2009) A randomized phase III trial in advanced endometrial carcinoma of surgery and volume directed radiation followed by cisplatin and doxorubicin with our without paclitaxel: a Gynecologic Oncology Group study. Gynecol Oncol 112:543–552
Gehrig PA, Bae-Jump VL (2010) Promising novel therapies for the treatment of endometrial cancer. Gynecol Oncol 116:187–194
Bokhman JV (1983) Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 15(1):10–17
Hecht J, Mutter GL (2006) Molecular and pathologic aspects of endometrial carcinogenesis. J Clin Oncol 24(29):4783–4791
Lax SF, Kendall B, Tashiro H et al (2000) The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer 88(4):814–824
Doll A, Abal M, Rigau M et al (2008) Novel molecular profiles of endometrial cancer-new light through old windows. J Steroid Biochem Mol Biol 108(3–5):221–229
Bansal N, Yendluri V, Robert M et al (2009) The molecular biology of endometrial cancers and the implications for pathogenesis, classification, and targeted therapies. Cancer Control 16(1):8–13
Vardi JR, Tadros GH, Anselmo MT et al (1992) The value of exploratory laparotomy in patients with endometrial carcinoma according to the new International Federation of Gynecology and Obstetrics staging. Obstet Gynecol 80:204–208
Ballon SC, Berman ML, Donaldson RC et al (1979) Pulmonary metastases of endometrial carcinoma. Gynecol Oncol 7:56–65
Bouros D, Papadakis K, Siafakas N et al (1996) Natural history of patients with pulmonary metastases from uterine cancer. Cancer 78:441–447
Doll A, Gonzalez M, Abal M et al (2009) An orthotopic endometrial cancer mouse model demonstrates a role for RUNX1 in distant metastasis. Int. J Cancer 125:257–263
O’Neill K, Lyons SK, Gallagher WM et al (2010) Bioluminescent imaging: a critical tool in pre-clinical oncology research. J Pathol 220:317–327
Zhang C, Yan Z, Arango ME et al (2009) Advancing bioluminescence imaging technology for the evaluation of anticancer agents in the MDA-MB-435-HAL-Luc mammary fat pad and subrenal capsule tumor models. Clin Cancer Res 15:238–246
Du X, Jiang T, Sheng X et al (2009) Inhibition of osteopontin suppresses in vitro and in vivo angiogenesis in endometrial cancer. Gynecol Oncol 115:371–376
Dai D, Holmes AM, Nguyen T et al (2005) A potential synergistic anticancer effect of paclitaxel and amifostine on endometrial cancer. Cancer Res 65(20):9517–9524
Saidi SA, Holland CM, Charnock-Jones S et al (2006) In vitro and in vivo effects of the PPAR-alpha agonists fenofibrate and retinoic acid in endometrial cancer. Mol Cancer 5:13
Wallace AE, Sales KJ, Catalano RD et al (2009) Prostaglandin F2A-F-prostanoid receptor signaling promotes neutrophil chemotaxis via chemokine (C-X-C Motif) Ligand 1 in endometrial adenocarcinoma. Cancer Res 69:5726–5733
Fidler IJ (1991) Orthotopic implantation of human colon carcinomas into nude mice provides a valuable model for the biology and therapy of metastasis. Cancer Metastasis Rev 10:229–243
John CM, Leffler H, Kahl-Knutsson B et al (2003) Truncated galectin-3 inhibits tumor growth and metastasis in orthotopic nude mouse model of human breast cancer. Clin Cancer Res 9:2374–2383
Chong L, Ru** Y, Jiancheng B et al (2006) Characterization of a novel transplantable orthotopic murine xenograft model of a human bladder transitional cell tumor (BIU-87). Cancer Biol Ther 5(4):394–398
Report Meeting (2009) The new FIGO staging system for cancers of the vulva, cervix, endometrium and sarcomas. Gynecol Oncol 115:325–328
Kamat AA, Merritt WM, Coffey D et al (2007) Clinical and biological significance of vascular endothelial growth factor in endometrial cancer. Clin Cancer Res 13:7487–7495
Lee JW, Stone RL, Lee SJ et al (2010) EphA2 targeted chemotherapy using an antibody drug conjugate in endometrial carcinoma. Cancer Res 16:2562–2570
Takahashi K, Saga Y, Mizukami H et al (2009) Cetuximab inhibits growth, peritoneal dissemination, and lymph node and lung metastasis of endometrial cancer, and prolongs host survival. Int J Oncol 35:725–729
Acknowledgments
The authors would like to thank Lisa Piccione for correction of the manuscript. This work has been supported by the Spanish Ministry of Science and Innovation (SAF 2005-06771; SAF 2008-03996; SAF 2010-10635-E; SAF2011-26548), the Spanish Ministry of Health (RTICC RD06/0020/0058, RD06/0020/1034; CP08/00142; PI08/0797), the Catalan Institute of Health (DURSI 2005SGR00553) and the Department of Universities and Research, Catalan Government (2009SGR00487, 2005SGR00553), the ACCIO program (RDITSCON07-1-0001), the Foundation La Marato de TV3 (grant 050431), the IV Grant Fundació Santiago Dexeus Font for Clinical Investigation Projects 2009, the National Programme of Biotechnology (FIT-010000-2007-26), and the European Commission Program Fondo Europeo de Desarrollo Regional (FEDER). M.LL. is recipient of a predoctoral fellowship from the Spanish Ministry of Innovation and Science (FI07/00423).
Author information
Authors and Affiliations
Corresponding author
Additional information
Silvia Cabrera, Marta Llauradó, Antonio Gil-Moreno and Jaume Reventós have contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10585_2011_9444_MOESM1_ESM.tif
Supplementary Figure 1. In vitro appearance and behavior of Hec-1A cell line and Hec-1A-Fluc clones. (A) Phase-contrast images and cell prolIferation assay of the Hec-1A cell line and clones 4, 5, 8 and 9 of the Hec-1A Fluc cells (20×). (B) Cell proliferation assay using the CellTiter 96® AQueous One Solution (MTS) (Promega) and cell invasion assay performed in triplicate using the Cytoselect 24-well cell migration assay (Cell Biolabs) for the clones 4, 5,8 and 9 of the Hec-1A Fluc cells. (C) Cell proliferation assay using the CellTiter 96® AQueous One Solution (MTS) (Promega) and cell invasion assay performed in triplicate using the Cytoselect 24-well cell migration assay (Cell Biolabs) for the non transfected Hec-1A cell line and the clone 5 of the Hec-1A Fluc cells, which was the selected clone to generate de murine model. (TIF 1.07 MB)
Rights and permissions
About this article
Cite this article
Cabrera, S., Llauradó, M., Castellví, J. et al. Generation and characterization of orthotopic murine models for endometrial cancer. Clin Exp Metastasis 29, 217–227 (2012). https://doi.org/10.1007/s10585-011-9444-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-011-9444-2