Abstract
Small membranes known as exosomes surround them and are released by several cell types both in vitro and in vivo. These membranes are packed with a variety of biomolecules, including proteins, lipids, deoxyribonucleic acid (DNA), ribonucleic acid (RNA), and non-coding RNA (ncRNA). As a source of biological nanomaterials, exosomes play a role in information and substance transmission between cells and have been identified as a general method of facilitating communication during interactions between the body, target organs, and toxins.. In order to understand the changes and mechanism of the composition and level of exosomes after biotoxin infection, this review focuses on current findings on the exosomes and highlights their novel uses in the toxicity mechanism. Exosomes are mainly used as a delivery carrier or mediated by receptors, and play an immune role after the toxin enters the body. This review expounds on the importance of exosomes in the toxicological mechanism of biotoxins and provides new insights for further diagnosis of toxic biomarkers, detoxification, and treatment development.
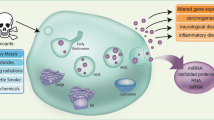
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exosomes are membranous vesicles released into the extracellular matrix after the fusion of intracellular poly vesicles and cell membranes (Kalluri and LeBleu 2020). Almost all types of cells produce and release exosomes. Exosomes are nano-scale lipid inclusion structures with a diameter of 30–150 nm, which are coated with lipids protein, DNA, RNA and ncRNA. Body fluids like blood, saliva, urine, and breast milk all contain exosomes, where they travel through the circulatory system to reach other cells and tissues, allowing for remote regulation (Zhang et al. 2015; Kalluri and LeBleu 2020).
Toxic natural substances (such as zearalenone, vomitoxin), also known as biotoxins, contaminate food, pet food, and livestock feed, posing risks to the environment and feed chain (including the aquaculture industry). Biotoxins are non-replicable toxic chemicals by living cells for self-defense (Xu et al. 2021). Although much effort has been invested in toxin research, the control and toxicological assessment of toxic secondary metabolites still face many problems. The agriculture, food, and feed industries are vulnerable to biotoxin contamination. In addition, studies on mechanisms of toxicity, especially chronic toxicity, remain insufficient. In more detail, the biotoxins' toxic mechanisms refer to their action patterns, toxicokinetics, adverse effects in humans and animals, including the combined effects of mixtures, and the interaction of these toxin mixtures at the organ and cellular level (Kordiš and Gubenšek 2000). Therefore, more research is required to completely understand the harmful consequences of biotoxins in the environment and food chain. more research is needed to fully comprehend the toxic effects of biotoxins in the environment and feed chain. Biotoxins typically exhibit a strong selective action on cell membranes, ion channels, receptors, ribosomal proteins, and other specific target molecules that participate in living systems and metabolic processes, resulting in varying degrees of lethal or toxic effects (Ménez 1998; Kordiš and Gubenšek 2000; Yuan et al. 2022).
Exosomes can cross biological barriers and participate in short and long-distance intercellular communication (He et al. 2018). However, there are no specific and validated biomarkers and mechanisms explaining how most biotoxins, such as mycotoxins, exert their effects after entering the body. In addition, exosomes act as biomarkers to provide unique information about the comprehensive toxicity of low-dose toxin mixtures. This makes it possible to accurately assess the health risks of exposure to biotoxin mixtures for both humans and animals. Based on existing research, this review aims to identify the toxic mechanism of biotoxins and summarize the research progress of exocrine in the toxicological mechanism of biotoxins, providing theoretical references for their toxicity mechanism, detoxification, and therapies.
Exosome
Exosomes are double-layer vesicles with a diameter of about 30-150 nm secreted by cells, which are packed with a large number of bioactive substances such as proteins, microRNA (miRNA), and long noncoding RNA (lncRNA) (Zhang et al. 2015; Fuchs et al. 2021). The exosomes widely exist in blood, urine, cerebrospinal fluid, saliva, and milk (Hariharan et al. 2021). Most eukaryotic cells secrete exosomes into the extracellular space, and these exosomes are vesicles that promote intercellular signal communication at the molecular and physiological levels (Cun et al. 2022).
As a new type of extracellular vesicles, exosomes are transported between cells by surface ligands or between cells. They are directly or indirectly involved in intercellular communication (David and Zimmermann 2020). When the external environment stimulates the body, corresponding tissue cells receive signals that either increase exosome production or inhibit exosome secretion. When exosomes are discharged and released outside the cell (David and Zimmermann 2020), they selectively package proteins, nucleic acids, cytokines, and other active substances from the donor cells. The contents are then released upon entering the recipient cells. Therefore, it can cause functional responses and alter recipient cell phenotypes, affecting their physiological states. Exosomes are associated with immune response, viral pathogenicity, pregnancy, cardiovascular disease, central nervous system-related diseases, and cancer (Doyle and Wang 2019). The proteins, metabolites, and nucleic acids delivered by exosomes to recipient cells can effectively alter their biological responses. This exosome-mediated response can exacerbate or alleviate disease (Hamzah et al. 2021). The intrinsic properties of exosomes in regulating complex intracellular pathways enhance their potential utility in the therapeutic potential of many diseases.
Engineering exosomes
Exosomes have been investigated for use as drug delivery systems for a variety of diseases due to their low immunogenicity, preference for tumor localization, endogenous and acquired targeting, and stability (Fig. 1) (Zhang et al. 2019; Shao et al. 2020). At present, most of the engineering exosomes on the market are modified by milk-derived and HEK293 cell-derived exosomes, and the exosomes of these two sources are relatively stable. Some hydrophobic or hydrophilic small-molecule drugs can be delivered to targeted locations by loading into exosomes (Yang et al. 2019; Hade et al. 2021). In general, exosomes can act as a delivery vehicle for small molecules, ultimately leading to a higher accumulation of the molecules in target cells, increasing the existence time of drugs in the blood circulation, improving the stability of small molecules, and thus increasing the therapeutic efficiency of small molecule drugs (Zhao et al. 2022). Duan et al. (Duan et al. 2021) used exosomes to encapsulate curcumin and delivered it to microglia by nasal delivery. Curcumin-coated exosomes were shown to be protective against lipopolysaccharide (LPS)-induced encephalitis, experimental autoimmune encephalitis, and inhibited myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis (Gurung et al. 2021). Plant organs also secrete exosomes (plant exosome-like nanovesicles, PELNVs), and plants are a cost-effective source of exosomes with high production performance (Zhang et al. 2018). Generally, they are extracted from edible plants, can be efficiently produced in large quantities, and can compensate for the limitations of mammal-derived exosomes, which have attracted more and more attention (Cao et al. 2023). At present, there are exosomes from grape (Teng et al. 2022), broccoli (Duan et al. 2023), ginger (Zhu and He 2023), carrot (Mu et al. 2014), and other sources (Record 2013), which are similar to general animal derived exosomes in terms of size distribution, morphology, and contents. They also have certain clinical applications. For example, Ju et al. (Ju et al. 2013) demonstrated that grapevine exosome-like nanoparticles (GELNs) could target intestinal stem cells. GELNs could penetrate the mouse intestinal mucus barrier and be taken up by intestinal stem cells. Lgr5hi intestinal stem cells were induced by the Wnt/β-catenin pathway. GELN treatment resulted in activation of the Wnt-mediated Tcf4 transcriptional machinery in the crypts, and regulates the proliferation of stem cells. The most widely used ginger exosomes have been verified to deliver anticancer drugs and inhibit tumor growth (Brahmbhatt et al. 2013). At the same time, Given exosomes extracted from ginger caused changes in intestinal miRNAs in mice with colitis. Combined analysis of intestinal flora showed that miRNAs regulated the level of intestinal microorganisms, thereby normalizing impaired intestinal function (Zhang et al. 2016).
Exosomes as diagnostic markers for disease
As exosomes reflect some of the characteristics of parental cells, exosomes secreted by tumor cells indicate the features of parental tumor cells (Fig. 1). Thus, analyzing the expression of exosome protein or RNA is a powerful tool for disease diagnosis (He et al. 2018). Exosomes improved the apoptosis of intestinal epithelial cells induced by vomitoxin (DON) and reversed the inhibitory effect of DON on the proliferation of intestinal epithelial cells. Further exploration revealed that miR221 and miR222 in intestinal epithelial cells were associated with apoptosis and proliferation of intestinal epithelial cells through the regulation of phosphatase with tensor protein homolog (PTEN) as a target gene. Moreover, the apoptosis and proliferation inhibition of intestinal epithelial cells induced by DON depends on PTEN, whereas miR221 and miR222 could improve intestinal epithelial cell damage by targeting PTEN (Khayambashi et al. 2021). In prostate cancer, two different exosome biomarkers, prostate cancer-associated 3 (PCA-3) and transmembrane serine protease 2 (TMPRSS2), are directly associated with incidence. Proteins derived from urine exosomes are diagnostic markers for urinary tract diseases and bladder cancer (Khayambashi et al. 2021). In various studies, exosome miRNAs have been shown to serve as biomarkers to diagnose renal fibrosis and cardiovascular diseases (Li et al. 2021). Compared with healthy individuals, the content of serum exosomes is slightly upregulated in patients with benign tumors while significantly increased in cancer patients (Liang et al. 2021). There are about 175 miRNAs commonly found in both tumor cells and exosomes, and the raised miRNAs in exosomes are distributed. The distribution of up-regulated miRNAs in exosomes matches the distribution of up-regulated miRNAs in ovarian cancer patients at different stages, but this method cannot completely distinguish different stages of cancer (Liu et al. 2018; Qiu et al. 2022). Therefore, it is crucial to develop relevant technologies that can enrich disease-related (such as tumor-derived) exosomes and identify their specific components, thereby significantly improving the sensitivity of exosomes as biomarkers (Sun et al. 2018a).
Biotoxins
Biotoxins, also known as biological toxins and natural toxins, are metabolites derived from various organisms (Clark et al. 2019; Panda et al. 2022). These harmful biological agents cannot reproduce within organisms and pose threats to other species. Due to the diversity of biological species, their corresponding metabolites, biotoxins, show diverse characteristics, such as source, chemical structure, and mechanism of action (Herzig et al. 2020). Some of them can cause ignorable toxic effects on biological organisms. Therefore, It is crucial to study the involvement of exosomes in the toxicity mechanisms of biotoxins. Biotoxins can be classified into animal toxins, marine toxins, phytotoxins, and microbial toxins based on their origin (Fig. 2).
Animal toxins
The evolution of animal toxins is closely inspired by the natural world. To protect themselves and hunt for prey, some terrestrial animal species secrete toxins and interfere with or destroy the physiological processes of other organisms through bioactive substances such as toxins themselves and gene sequences, thus affecting the natural environment, affecting the natural environment, and pollute food and livestock feed through the food chain, and then harming human health (Zhang 2015; Kozlov 2018; Yu et al. 2020). Animal biotoxins are primarily produced by the venomous glands of venomous animals (Zhao et al. 2019). Most of them are protein compounds with peptide bonds and disulfide bonds (Kordiš and Gubenšek 2000). They target specific organisms through protein sites or ion channels by binding to receptors and play their role in disrupting physiological processes (Ménez 1998; Yuan et al. 2022), resulting in hematologic toxicity, neurotoxicity, and cytotoxicity. Common animal toxins include hydrocarbons, heterocyclic compounds, alkaloids, biogenic amines, peptides, and proteins, such as snake venom, bee venom, and scorpion venom (Sukumwang and Umezawa 2013; Undheim et al. 2016; Waheed et al. 2017; Walker 2020; Johannes 2022).
Marine toxins
Marine toxins are different from animal toxins. Marine biotoxins refer specifically to the toxic metabolism of marine organisms, which include algae, shellfish, coelomatids, and some tropical coral reef fish (Salzman et al. 2006). They are the most widely distributed and most toxic biotoxins (Halstead 1981). Marine biotoxins can be divided into three categories based on their chemical structures: peptide toxoids, polyether toxoids, and alkaloid toxoids. Marine toxins exhibit high selectivity for receptors. They typically act on nerves and muscles to stimulate specific targets on cell membranes, such as neuroreceptors or ion channels, thereby affecting a range of receptor-related cellular regulatory activities.(Van Dolah 2000; Leal and Cristiano 2022). Also, they exhibit a wide range of toxicity in the nervous, cardiovascular, and cytotoxic systems (Ye et al. 2019).
Phytotoxins
Competitive evolution between plants and herbivores forced plants to produce a wide variety of toxic secondary metabolites to protect themselves, and these secondary metabolites are known as phytotoxins (Strobel 1982; Adamski et al. 2021). However, phytotoxins inhibit plant growth and harm herbivores and humans above the maximum tolerated doses (Bucheli 2014). Phytotoxins have no common structural characteristics, and they come in different categories, such as non-protein amino acids, peptides, proteins, alkaloids, and glycosides or combinations of these categories (Eberhard and Ulrike). Aconitoid alkaloids, brucine, and muscarine are common plant toxins. When herbivores consume phytotoxins, the toxins are transported to their gastrointestinal tract, attack cells, and inhibit critical cell function (Finkelstein et al. 2010). However, the animal's body itself catalyzes the degradation of toxins through specific enzymes to alter their molecular structure, such as malonylation of phenolic glycosides, thereby reducing their toxicity. Also, some hydrolases can degrade the activity of enzymes and interact with each other to chelate toxic secondary metabolites produced by plants (Boeckler et al. 2011).
Microbial toxins
The vast majority of foodborne diseases result from microbial toxins, which are produced during microbial growth and reproduction. They are secondary toxic biotoxins second only to marine toxins. According to sources, they can be classfied into two categories: mycotoxins and bacterial toxins (Schmitt et al. 1999; Pleadin et al. 2019; **ong et al. 2022). In more detail, common mycotoxins include aflatoxin, ochratoxin, and zearalenone (Alshannaq and Yu 2017). Bacterial toxins are generally two-component protein toxins and LPS endotoxins, such as botulinum toxin, cholera toxin, and enterotoxin (Faïs et al. 2018; Lemichez et al. 2020). Animal tissues, such as the liver, kidney, or gastrointestinal tract, can metabolize these toxins and generate secondary toxic metabolites, leading to liver and kidney toxicity and gastrointestinal dysfunction (Pier 1992; Alshannaq and Yu 2017). Therefore, attention is paid not only to the toxins themselves but also to their metabolite in the study of microbial toxins (Stone and Darlington 2017).
The toxic effects of biotoxins work through various mechanisms, such as inducing oxidative damage and apoptosis, and exosomes usually transmit signals through this general pathway. Moreover, oxidative stress is the production of large amounts of free radicals, such as reactive oxygen species (ROS) by the body, leading to a variety of harmful stimuli. Untimely free radical scavenging leads to the imbalance of oxidative and antioxidant systems in the body and causes oxidative damage to tissues and cells (Lai et al. 2022). As one of the initial signals produced after cell stress, ROS is critical in the toxic mechanism of biotoxins by lipid peroxidation, reducing the activity of antioxidant enzymes and inducing DNA damage (Liang et al. 2021). In contrast, intracellular ROS increases the number of MVBs by inhibiting their degradation in lysosomes, thereby enhancing the release of exosomes under the synergistic effect of glutathione (GSH) depletion on the external surface that can be regulated by oxidative stress (Liu et al. 2018). Higher ROS levels can reduce the production of exosomes by activating cell autophagy to degrade MVBs. Hypoxia-inducible factor (HIF) and exosomes are involved in the immunotoxicity of trichothecenes (T-2 toxin). T-2 toxin triggers cellular hypoxia, while mitochondria produce abundant ROS under hypoxic conditions and activate Hypoxia-inducible factor 1 subunit alpha (HIF-1α) signaling. The release of exosomes is related to HIF-1α and controls the adaptive physiological response to hypoxia through the NF-κB pathway (Wu et al. 2020). In addition, Milane et al. (Milane et al. 2015) demonstrated that the regulation of exosome release is dependent on the GSH level on the external surface regulated by oxidative stress. Likewise, cigarette smoke extract (CSE) caused oxidative stress and enhanced the release of exosomes from human bronchial epithelial cells (Noonin and Thongboonkerd 2021).
The mechanism of toxin-induced cellular exosomes is unique from the others. For instance, OTA-induced cytotoxicity is partly delivered by EXO-OTA. This exosome leads to cell cycle arrest and alters protein metabolism, resulting in cytotoxicity, which can further worsen developmental delays and other diseases (Zhu et al. 2021). Toxin-treated nasopharyngeal carcinoma cells produce exosomes containing latent membrane protein-1 (LMP1). Consequently, neighboring cells uptake these exosomes and alter their transcriptional profile (Yang et al. 2019). The transcriptional changes through LMP1-mediated release of epidermal growth factor receptor from exosomes further result in the activation of ERK and PI3K/Akt pathways in epithelial cells, endothelial cells, and fibroblasts. These pathways are well-known for promoting cell growth and migration (Yang et al. 2017). After the body is exposed to the toxin, the secretion of exosome often increased or decreased. The most studied is that the level of miRNA contained in exosomes will also increase or decrease accordingly, and then cause changes in the genetic environment. In addition, exosomes can also contain toxins under special circumstances, which can cause further cytotoxicity. For example, exosomes released from Stx2a-treated human macrophage like cells are even more cytotoxic to HK-2 cells than toxin exposure alone. In addition, some exosomes as a key part of oxidative stress play a role through the expression level of the contained substances, affecting the body's resistance to toxins, or further deepening the damage of the body.
Exosomes as biotoxin toxicity signal transmitters
Biotoxins disrupt intercellular signal transduction, induce oxidative damage and apoptosis, and exhibit high toxicity to the kidney, liver, nerve, and reproductive systems (Yang et al. 2018; Liu et al. 2021; Huang et al. 2022). Therefore, exosomes significantly contribute to the toxic process of biotoxins. At the molecular level, the expression of exosomes on receptor cells and cell surface receptors varies according to the toxin type and target cells (Table 1). Existing studies found that this functional heterogeneity enables the exosomes to induce cell survival, apoptosis, or immune regulation. All these features result in a higher level of complexity and heterogeneity in the toxic effects of biotoxins (Joyce et al. 2016).
Receptor-mediated
Exosomes directly activate receptors on the surface of target cells through protein molecules or lipid ligands, generate signaling complexes, and activate intracellular signaling pathways (Fig. 3). This effect will cause recipient cells to secrete more or less exosomes after stimulation after stimulation directly, thereby further expanding the intracellular influence. Cholera toxin (CT) activates the intracellular adenylate kinase of target cells directly through an exosome-mediated cell communication pathway. It has also been found that CT can be propagated in biologically active form through exosomes and then transferred in a typical endoplasmic reticulum retrograde pathway under the action of heat shock proteins 90 (HSP90) and protein co-enzyme protein disulfide isomerase (PDI). It can extend the pathophysiological effects from the initial host cells, such as the gut, to a variety of cells (Zanetti et al. 2018). When human A594 cells were exposed to diphtheria toxin, Autophagy-related (ATG) proteins reduced ADAM10 on the cell surface through lysosomal and proteasome-independent processes was observed. Consequently, the cells secreted more exosomes to protect themselves from diphtheria toxin by directly inducing toxin oligomerization on the membrane (Keller et al. 2020). Generally, this direct effect is applicable when the toxins have a protein structure or target specific cells.
Vehicle of delivery
Exosomes can blend with the plasma membrane of cells or endocytose directly into the cells, carrying proteins, lipids, and other active molecules into the cells, thereby regulating the function and biological behavior of cells (Fig. 4). When exposed to external stimuli, exosomes deliver small RNAs to play a great concern role in reducing endotoxin and achieving homeostasis in the body. Alexander et al. (Alexander et al. 2015) injected exosomes containing miR-146a and miR-155 into LPS-treated mice and found that these miRNAs were delivered to various tissues, where they inhibited inflammatory-related gene targets and regulated inflammatory responses to endotoxin. circANTXR1 exists in the exosomes of liver cancer cells exposed to toxins. Exosome circANTXR1 overexpression promotes HCCLM3 cell proliferation and metastasis. Also, exosomes are participated in the intercellular transport process of circANTXR1, thereby affecting the biological function of HCC cells (Huang et al. 2021).
Exosomes have the potential as carriers to facilitate signal transduction between toxins. Since most exosomes are secreted by cells, exosomes change accordingly with biotoxins-induced endotoxin and cell apoptosis (Noonin and Thongboonkerd 2021). Exosomes from Stx2 (a second type of Shiga toxin)-treated THP-1 cells contained Stx2 and were phagocytosed by Gb3-positive cells, in which process they selectively induced Gb3-dependent apoptosis associated with Caspase3/7 activation. At the same time, the secreted exosomes Stx2-Exo contain a large amount of Exo-mRNA encoding inflammatory cytokines IL-1β, which aggravate the pro-inflammatory state of the receptor cells. Also, Stx2-Exo affects biomarkers concerning endoplasmic reticulum stress and MAPK pathway (Lee et al. 2020).
Regulation of immune response
The role of exosomes in immune regulation can be summarized as antigen presentation, regulation of host immune response, expression of some activation molecules or complement factors to cause immune surveillance, enhance tumor cell invasion, and mediate intercellular communication (Shao et al. 2020) (Fig. 5). Toxin-induced interactions in vivo involve complex biological processes. The presence of toxins can stimulate cells to produce exosomes, which are enriched in inflammation-related signaling molecules, or to recruit surrounding signaling molecules such as cytokines and chemokines (MHC-I and MHC-II). When they are taken up by adjacent or distant cells, they can activate or enhance the inflammatory response of these cells, thereby promoting the body's immune response to the toxin. Colchicine prevents the migrations of immune cells by inhibiting intracellular proinflammatory cytokines, vesicle trafficking, and exosomes release at the site of inflammation (Jerschke et al. 2023). Generally, various immune cells (e.g. dendritic cells, lymphocytes) have been expounded to release immunomodulatory exosomes. βγ-CAT isolated from the skin secretions of B.maxima (a frog species) stimulates the production and release of functional exosomes in mice, enhances the antigen-presenting ability of mouse bone marrow-derived immune cells, and activates T cell responses by secreting exosomes. Furthermore, these findings indicated that βγ-CAT transport membrane integration proteins and immune factors (MHC-I and MHC-II) between cells through vesicle transportation and the release of exosomes (Deng et al. 2020). Besides, exosomes exhibit unique, innate immune responses against bacterial infection as bait to entrap membrane-acting virulence factors, such as pore-making toxins, and protect target tissues (Sun et al. 2019). These "deplastids" act similarly to engineered exosomes, which neutralize toxins from Gram-positive bacteria. In addition, ATG proteins modulate Gram-positive bacteria-induced exosome production during host defense. This mechanism is different from the previously reported role of ATG16L1 in promoting plasma membrane repair during Listeria monocytogenes infection (Keller et al. 2020). Another interesting virulence mechanism was proposed by identifying important immune factors (IL6, caspase-1, IL-1b, IL-18, and IL-33) in exosomes released by EBV-infected cells (Sun et al. 2018b). In the innate immune sensor STING (IFN gene stimulator), miRNA and mRNA are exported by exosomes, which are then delivered to uninfected cells (Wang et al. 2023). The specific mechanism and functional significance of this process are currently unclear and require further study.
It can be concluded that exosomes play a dual role in the regulation of the immune system through either the toxin-induced body responses, which act as host programs to induce innate and adaptive immunity, or as evading strategies to eliminate inflammation (Wu et al. 2018).
Conclusion and prospect
Exosomes are critical vehicles of biotoxins. They promote toxin infection and host immunity in terms of toxicity mechanism (Meldolesi 2018). Exosomes are derived from various cell species and contain multiple contents, which endow them with heterogeneity, providing sufficient biological functions and space for artificial transformation (Kimiz-Gebologlu and Oncel 2022). This paper reviews the role of exosomes as a medium in the toxicological mechanism of biotoxins. The role of exosomes in microbial toxins has been studied by many people, and animal toxins and phytotoxins are slightly involved (Bignell et al. 2018; Gao et al. 2021). Nevertheless, there are still many unanswered questions including (1) the role of exosomes in marine toxins remains to be explored, (2) there is no close connection between exosomes and general mechanisms such as oxidative stress caused by toxins, (3) the question of whether exosomes play different roles after exposure to different doses of the toxin and what the consequences of their expression are still to be addressed (Table 2). In order to make exosomes widely used in the field of biotoxins and make progress as much as possible.
At present, the difficulty of research on exosomes is still in the aspect of extraction (Wu et al. 2019). The understanding of exosome physiology, diversity, internalization and molecular cargo transportation is still very limited, and the extraction is limited to ultracentrifugation and other methods (Ludwig et al. 2019; Pegtel and Gould 2019). A series of problems still need to be solved. For example, the diameter of exosomes is similar to that of some fungal bacteria. Fungi and other microorganisms are inevitable in biotoxin research, and it is difficult to exclude the contamination of microbial particles by differential ultracentrifugation (Sadeghi et al. 2023). However, the combination of polyethylene glycol treatment and ultracentrifugation fulfills the need to purify exosomes from viruses, they can also be applied to the study of exosome-mediated biotoxins. Future research should consider the potential effects of more carefully, for example, whether the secretion process of the exosome is the same as that of ordinary cells, and whether other metabolites will be produced, whether the exosome will transmit signals as a carrier in the process of detoxification and detoxification of the toxin still needs to be further studied. Because exosomes can be secreted by blood, urine and other body fluids, they provide a non-invasive detection possibility. In the early stage of toxin exposure, the health level of the body can be judged by detecting the content of exosomes in blood and urine or the level of special markers, which is of great significance for improving the acceptance of patients and the convenience of detection. However, at present, these applications exist in the development of diseases, and their applications in toxins are less, which are worthy of further development. With the development of isolation and purification technology, the focus has gradually shifted from lab research to clinical application. We believe that exosomes can contribute to establishing novel diagnosis and detoxification approaches for biotoxins.
Data availability
Data availability is not applicable to this article as no new data were created or analyzed in this study. The data that support the findings of this study are available from the corresponding author, **aoyun He, Kunlun Huang (Email: hexiaoyun@cau.edu.cn; foodsafety66@cau.edu.cn), upon reasonable request.
References
Abrami L, Brandi L, Moayeri M, Brown MJ, Krantz BA, Leppla SH, van der Goot FG. Hijacking multivesicular bodies enables long-term and exosome-mediated long-distance action of anthrax toxin. Cell Rep. 2013;5:986–96.
Adamski Z, Bufo SA, Milella L, Scrano L. Identification and functional characterization of plant toxins. Toxins (Basel). 2021;13:228.
Alexander M, Hu R, Runtsch MC, Kagele DA, Mosbruger TL, Tolmachova T, Seabra MC, Round JL, Ward DM, O’Connell RM. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commun. 2015;6:7321.
Alshannaq A, Yu JH. Occurrence, toxicity, and analysis of major mycotoxins in food. Int J Environ Res Pub Health. 2017;14(6):632.
Bignell DRD, Cheng Z, Bown L. The coronafacoyl phytotoxins: structure, biosynthesis, regulation and biological activities. Antonie Van Leeuwenhoek. 2018;111:649–66.
Boeckler GA, Gershenzon J, Unsicker SB. Phenolic glycosides of the salicaceae and their role as anti-herbivore defenses. Phytochemistry. 2011;72:1497–509.
Brahmbhatt M, Gundala SR, Asif G, Shamsi SA, Aneja R. Ginger phytochemicals exhibit synergy to inhibit prostate cancer cell proliferation. Nutr Cancer. 2013;65:263–72.
Bucheli TD. Phytotoxins: environmental micropollutants of concern? Environ Sci Technol. 2014;48:13027–33.
Cao M, Diao N, Cai X, Chen X, **ao Y, Guo C, Chen D, Zhang X. Plant exosome nanovesicles (PENs): green delivery platforms. Mater Horiz. 2023;10:3879–94.
Clark GC, Casewell NR, Elliott CT, Harvey AL, Jamieson AG, Strong PN, Turner AD. Friends or foes? emerging Impacts of biological toxins. Trends Biochem Sci. 2019;44:365–79.
Cun Y, ** Y, Wu D, Zhou L, Zhang C, Zhang S, Yang X, Zuhong W, Zhang P. Exosome in crosstalk between inflammation and angiogenesis: a potential therapeutic strategy for stroke. Mediators Inflamm. 2022;2022:7006281.
David G, Zimmermann P. Heparanase involvement in exosome formation. Adv Exp Med Biol. 2020;1221:285–307.
Deng CJ, Liu L, Liu LZ, Wang QQ, Guo XL, Lee WH, Li SA, Zhang Y. A secreted pore-forming protein modulates cellular endolysosomes to augment antigen presentation. FASEB J: Off Pub Fed Amer Soc Exp Biol. 2020;34:13609–25.
Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis.Cells. 2019;8(7):727.
Duan L, Xu L, Xu X, Qin Z, Zhou X, **ao Y, Liang Y, **a J. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale. 2021;13:1387–97.
Duan T, Wang X, Dong X, Wang C, Wang L, Yang X, Li T. Broccoli-derived exosome-like nanoparticles alleviate loperamide-induced constipation, in correlation with regulation on gut microbiota and tryptophan metabolism. J Agric Food Chem. 2023;71:16568–80.
Faïs T, Delmas J, Barnich N, Bonnet R, Dalmasso G. Colibactin: more than a new bacterial toxin. Toxins (Basel). 2018;10(4):151.
Finkelstein Y, Aks SE, Hutson JR, Juurlink DN, Nguyen P, Dubnov-Raz G, Pollak U, Koren G, Bentur Y. Colchicine poisoning: the dark side of an ancient drug. Clin Toxicol. 2010;48:407–14.
Fuchs A, Samovski D, Smith GI, Cifarelli V, Farabi SS, Yoshino J, Pietka T, Chang S-W, Ghosh S, Myckatyn TM, Klein S. Associations among adipose tissue immunology, inflammation, exosomes and insulin sensitivity in people with obesity and nonalcoholic fatty liver disease. Gastroenterology. 2021;161:968-981.e912.
Gao HN, Hu H, Wen PC, Lian S, **e XL, Song HL, Yang ZN, Ren FZ. Yak milk-derived exosomes alleviate lipopolysaccharide-induced intestinal inflammation by inhibiting PI3K/AKT/C3 pathway activation. J Dairy Sci. 2021;104:8411–24.
Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021;19:47.
Hade MD, Suire CN, Suo Z. Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells. 2021;10(8):1959.
Halstead BW. Current status of marine biotoxicology—an overview. Clin Toxicol. 1981;18:1–24.
Hamzah RN, Alghazali KM, Biris AS, Griffin RJ. Exosome traceability and cell source dependence on composition and cell-cell cross talk. Int J Molec Sci. 2021;22(10):5346.
Hariharan H, Kesavan Y, Raja NS. Impact of native and external factors on exosome release: understanding reactive exosome secretion and its biogenesis. Mol Biol Rep. 2021;48:7559–73.
He C, Zheng S, Luo Y, Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8:237–55.
Herzig V, Cristofori-Armstrong B, Israel MR, Nixon SA, Vetter I, King GF. Animal toxins - Nature’s evolutionary-refined toolkit for basic research and drug discovery. Biochem Pharmacol. 2020;181:114096.
Huang C, Yu W, Wang Q, Huang T, Ding Y. CircANTXR1 Contributes to the malignant progression of hepatocellular carcinoma by promoting proliferation and metastasis. J Hepatocell Carcinoma. 2021;8:1339–53.
Huang Y, Zhu Z, Luo C, Ma C, Zhu L, Kong L, Li R, Wu J, Yuan Z, Yi J. Betulinic acid attenuates cognitive dysfunction, oxidative stress, and inflammation in a model of T-2 toxin-induced brain damage. Environ Sci Pollut Res Int. 2022;29:52098–110.
Jerschke E, Barkovsky M, Jung N, Neuberger H, Stenzel J, Eyer F, Skerra A, Geith S. In vivo neutralization of colchicine toxicity by a PASylated anticalin in a rat model. Toxicology. 2023;492:153526.
Johannes L. Application of protein toxins as cell biological and pharmacological tools. Toxins. 2022;14.
Joyce DP, Kerin MJ, Dwyer RM. Exosome-encapsulated microRNAs as circulating biomarkers for breast cancer. Int J Cancer. 2016;139:1443–8.
Ju S, Mu J, Dokland T, Zhuang X, Wang Q, Jiang H, **ang X, Deng ZB, Wang B, Zhang L, Roth M, Welti R, Mobley J, Jun Y, Miller D, Zhang HG. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Molec Ther: J Amer Soc Gene Ther. 2013;21:1345–57.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478).
Keller MD, Ching KL, Liang F-X, Dhabaria A, Tam K, Ueberheide BM, Unutmaz D, Torres VJ, Cadwell K. Decoy exosomes provide protection against bacterial toxins. Nature. 2020;579:260–4.
Khayambashi P, Iyer J, Pillai S, Upadhyay A, Zhang Y, Tran SD. Hydrogel encapsulation of mesenchymal stem cells and their derived exosomes for tissue engineering. Int J Molec Sci. 2021;22(2):684.
Kimiz-Gebologlu I, Oncel SS. Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release: Off J Control Release Soc. 2022;347:533–43.
Kordiš D, Gubenšek F. Adaptive evolution of animal toxin multigene families. Gene. 2000;261:43–52.
Kozlov S. Animal toxins for channelopathy treatment. Neuropharmacol. 2018;132:83–97.
Lai, JJ, Chau ZL, Chen SY, Hill JJ, Korpany KV, Liang NW, Lin LH, Lin YH, Liu JK, Liu YC, Lunde R, Shen WT. Exosome processing and characterization approaches for research and technology development. Adv Sci. 2022;9:e2103222.
Leal JF, Cristiano MLS. Marine paralytic shellfish toxins: chemical properties, mode of action, newer analogues, and structure-toxicity relationship. Nat Prod Rep. 2022;39:33–57.
Lee KS, Lee J, Lee P, et al. Exosomes released from Shiga toxin 2a-treated human macrophages modulate inflammatory responses and induce cell death in toxin receptor expressing human cells. Cell Microbiol. 2020;22(11):e13249.
Lemichez E, Popoff MR, Satchell KJF. Cellular microbiology: Bacterial toxin interference drives understanding of eukaryotic cell function. Cell Microbiol. 2020;22:e13178.
Li B, Cao Y, Sun M, Feng H. Expression, regulation, and function of exosome-derived miRNAs in cancer progression and therapy. FASEB J: Off Pub Fed Amer Soc Exp Biol. 2021;35:e21916.
Liang Y, Duan L, Lu J, **a J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–95.
Liu C, Xu X, Li B, Situ B, Pan W, Hu Y, An T, Yao S, Zheng L. Single-exosome-counting immunoassays for cancer diagnostics. Nano Lett. 2018;18:4226–32.
Liu Y, Dong R, Yang Y, **e H, Huang Y, Chen X, Wang D, Zhang Z. Protective effect of organic selenium on oxidative damage and inflammatory reaction of rabbit kidney induced by T-2 toxin. Biol Trace Elem Res. 2021;199:1833–42.
Ludwig N, Whiteside TL, Reichert TE. Challenges in Exosome Isolation and Analysis in Health and Disease. Int J Mol Sci. 2019;20(19):4684.
Meldolesi J. Exosomes and ectosomes in intercellular communication. Curr Biol: CB. 2018;28:R435-r444.
Ménez A. Functional architectures of animal toxins: a clue to drug design? Toxicon. 1998;36:1557–72.
Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. J Control Release: Off J Control Release Soc. 2015;219:278–94.
Möller N, Ziesemer S, Hentschker C, Völker U, Hildebrandt J-P. Major determinants of airway epithelial cell sensitivity to s. aureus alpha-toxin: disposal of toxin heptamers by extracellular vesicle formation and lysosomal degradation. Toxins (Basel).2021;13:173.
Mu J, Zhuang X, Wang Q, Jiang H, Deng ZB, Wang B, Zhang L, Kakar S, Jun Y, Miller D, Zhang HG. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res. 2014;58:1561–73.
Noonin C, Thongboonkerd V. Exosome-inflammasome crosstalk and their roles in inflammatory responses. Theranostics. 2021;11:4436–51.
Panda D, Dash BP, Manickam S, Boczkaj G. Recent advancements in LC-MS based analysis of biotoxins: present and future challenges. Mass Spectrom Rev. 2022;41:766–803.
Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514.
Pier AC. Major biological consequences of aflatoxicosis in animal production. J Anim Sci. 1992;70:3964–7.
Pleadin J, Frece J, Markov K. Mycotoxins in food and feed. Adv Food Nutr Res. 2019;89:297–345.
Qiu S, **e L, Lu C, Gu C, **a Y, Lv J, Xuan Z, Fang L, Yang J, Zhang L, Li Z, Wang W, Xu H, Li B, Xu Z. Gastric cancer-derived exosomal miR-519a-3p promotes liver metastasis by inducing intrahepatic M2-like macrophage-mediated angiogenesis. J Exp Clin Cancer Res: CR. 2022;41:296.
Record M. Exosome-like nanoparticles from food: protective nanoshuttles for bioactive cargo. Molec Ther: J Amer Soc Gene Ther. 2013;21:1294–6.
Sadeghi S, Tehrani FR, Tahmasebi S, Shafiee A, Hashemi SM. Exosome engineering in cell therapy and drug delivery. Inflammopharmacology. 2023;31:145–69.
Salzman M, Madsen JM, Greenberg MI. Toxins: bacterial and marine toxins. Clin Lab Med. 2006;26(397–419):ix.
Schmitt CK, Meysick KC, O’Brien AD. Bacterial toxins: friends or foes? Emerg Infect Dis. 1999;5:224–34.
Shao J, Zaro J, Shen Y. Advances in exosome-based drug delivery and tumor targeting: from tissue distribution to intracellular fate. Int J Nanomed. 2020;15:9355–71.
Stone TW, Darlington LG. Microbial carcinogenic toxins and dietary anti-cancer protectants. Cell Molec Life Sci: CMLS. 2017;74:2627–43.
Strobel GA. Phytotoxins. Annu Rev Biochem. 1982;51:309–33.
Sukumwang N, Umezawa K. Earthworm-derived pore-forming toxin lysenin and screening of its inhibitors. Toxins. 2013;5:1392–401.
Sun Z, Shi K, Yang S, Liu J, Zhou Q, Wang G, Song J, Li Z, Zhang Z, Yuan W. Effect of exosomal miRNA on cancer biology and clinical applications. Mol Cancer. 2018;17:147.
Sun Z, Yang S, Zhou Q, Wang G, Song J, Li Z, Zhang Z, Xu J, **a K, Chang Y, Liu J, Yuan W. Emerging role of exosome-derived long non-coding RNAs in tumor microenvironment. Mol Cancer. 2018;17:82.
Sun W, Li Z, Zhou X, Yang G, Yuan L. Efficient exosome delivery in refractory tissues assisted by ultrasound-targeted microbubble destruction. Drug Delivery. 2019;26:45–50.
Teng Y, He J, Zhong Q, Zhang Y, Lu Z, Guan T, Pan Y, Luo X, Feng W, Ou C. Grape exosome-like nanoparticles: A potential therapeutic strategy for vascular calcification. Front Pharmacol. 2022;13:1025768.
Undheim EA, Mobli M, King GF. Toxin structures as evolutionary tools: Using conserved 3D folds to study the evolution of rapidly evolving peptides. BioEssays: News Rev Molec Cell Dev Biol. 2016;38:539–48.
Van Dolah FM. Marine algal toxins: origins, health effects, and their increased occurrence. Environ Health Perspect. 2000;108(Suppl 1):133–41.
Waheed H, Moin SF, Choudhary MI. Snake Venom: From Deadly Toxins to Life-saving Therapeutics. Curr Med Chem. 2017;24:1874–91.
Walker AA. The evolutionary dynamics of venom toxins made by insects and other animals. Biochem Soc Trans. 2020;48:1353–65.
Wang Y, Ren L, Bai H, ** Q, Zhang L. Exosome-autophagy crosstalk in enveloped virus infection. Int J Molec Sci. 2023;24.
Watanabe-Takahashi M, Yamasaki S, Murata M, Kano F, Motoyama J, Yamate J, Omi J, Sato W, Ukai H, Shimasaki K, Ikegawa M, Tamura-Nakano M, Yanoshita R, Nishino Y, Miyazawa A, Natori Y, Toyama-Sorimachi N, Nishikawa K. Exosome-associated Shiga toxin 2 is released from cells and causes severe toxicity in mice. Sci Rep. 2018;8:10776.
Wu P, Zhang B, Shi H, Qian H, Xu W. MSC-exosome: a novel cell-free therapy for cutaneous regeneration. Cytotherapy. 2018;20:291–301.
Wu Q, Qin Z, Kuca K, You L, Zhao Y, Liu A, Musilek K, Chrienova Z, Nepovimova E, Oleksak P, Wu W, Wang X. An update on T-2 toxin and its modified forms: metabolism, immunotoxicity mechanism, and human exposure assessment. Arch Toxicol. 2020;94:3645–69.
Wu X, Showiheen SAA, Sun AR, Crawford R, **ao Y, Mao X, Prasadam I. Exosomes extraction and identification. Methods Molec Biol 2019;2054:81–91.
**ong X, Tian S, Yang P, Lebreton F, Bao H, Sheng K, Yin L, Chen P, Zhang J, Qi W, Ruan J, Wu H, Chen H, Breault DT, Wu H, Earl AM, Gilmore MS, Abraham J, Dong M. Emerging enterococcus pore-forming toxins with MHC/HLA-I as receptors. Cell. 2022;185:1157-1171.e1122.
Xu X, Li T, Ji Y, Jiang X, Shi X, Wang B. Origin, succession, and control of biotoxin in wine. Front Microbiol. 2021;12:703391.
Yang JY, Zhang YF, Li YX, Meng XP, Bao JF. l-arginine protects against oxidative damage induced by T-2 toxin in mouse Leydig cells. J Biochem Mol Toxicol. 2018;32:e22209.
Yang F, Liao X, Tian Y, Li G. Exosome separation using microfluidic systems: size-based, immunoaffinity-based and dynamic methodologies. Biotechnol J. 2017;12.
Yang B, Chen Y, Shi J. Exosome biochemistry and advanced nanotechnology for next-generation theranostic platforms. Adv Mater. 2019;31:e1802896.
Ye W, Liu T, Zhang W, Zhu M, Liu Z, Kong Y, Liu S. Marine toxins detection by biosensors based on aptamers. Toxins. 2019;12.
Yu C, Yu H, Li P. Highlights of animal venom research on the geographical variations of toxin components, toxicities and envenomation therapy. Int J Biol Macromol. 2020;165:2994–3006.
Yuan FC, Sun FD, Zhang L, Huang B, An HL, Rong MQ, Du CW. General mechanism of spider toxin family I acting on sodium channel Nav1.7. Zool Res. 2022;43:886–96.
Zanetti C, Gallina A, Fabbri A, Parisi S, Palermo A, Fecchi K, Boussadia Z, Carollo M, Falchi M, Pasquini L, Fiani ML, Sargiacomo M. Cell propagation of cholera toxin CTA ADP-ribosylating factor by exosome mediated transfer. 2018;19:1521.
Zhang F, Sun S, Feng D, Zhao W-L, Sui S-F. A novel strategy for the invasive toxin: Hijacking exosome-mediated intercellular trafficking. Traffic. 2009;10:411–24.
Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinforma. 2015;13:17–24.
Zhang M, Viennois E, Prasad M, Zhang Y, Wang L, Zhang Z, Han MK, **ao B, Xu C, Srinivasan S, Merlin D. Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–40.
Zhang H, Wang L, Li C, Yu Y, Yi Y, Wang J, Chen D. Exosome-induced regulation in inflammatory bowel disease. Front Immunol. 2019;10:1464.
Zhang Y. Why do we study animal toxins? Dong wu xue yan jiu. Zool Res. 2015;36:183–222.
Zhang Z, **ng T, Chen Y, **ao J. Exosome-mediated miR-200b promotes colorectal cancer proliferation upon TGF-β1 exposure. Biomed Pharmacotherapy Biomed Pharmacotherapie 2018;106:1135–1143.
Zhao Y, Chen Z, Cao Z, Li W, Wu Y. Defensins, a novel type of animal toxin-like potassium channel inhibitor. Toxicon: Off J Int Soc Toxinol. 2019;157:101–5.
Zhao L, Wang H, Fu J, Wu X, Liang XY, Liu XY, Wu X, Cao LL, Xu ZY, Dong M. Microfluidic-based exosome isolation and highly sensitive aptamer exosome membrane protein detection for lung cancer diagnosis. Biosens Bioelectron. 2022;214:114487.
Zhu H, He W. Ginger: a representative material of herb-derived exosome-like nanoparticles. Front Nutr. 2023;10:1223349.
Zhu L, Wang H, Yuhan J, Zhang B, Li H, Asakiya C, Huang K, He X, Xu W. Exosomes mediated the delivery of ochratoxin A-induced cytotoxicity in HEK293 cells. Toxicology. 2021;461:152926.
Funding
This work was supported by grants from the Chinese National Natural Science Foundation (General Program, No.32372442) to Kunlun Huang.
Author information
Authors and Affiliations
Contributions
Writing – Original Draft Preparation, T.X.X.; Language issues, B.X.H.F.; Writing – Review & Editing, X.Y.H, K.L.H.; All authors contributed to manuscript revision, read, and approved the sub-mitted version. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, T., Huangfu, B., He, X. et al. Exosomes as mediators of signal transmitters in biotoxins toxicity: a comprehensive review. Cell Biol Toxicol 40, 27 (2024). https://doi.org/10.1007/s10565-024-09867-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10565-024-09867-4