Abstract
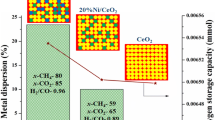
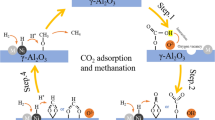
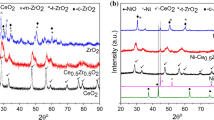
Hydrogen production from methane with CO2 utilization over exsolution derived NiCu/CeO2 catalysts was studied. To form highly dispersed supported bimetallic NiCu particles the Ce0.75(NiCu)0.25O1.75 catalyst precursors (Cu/Ni molar ratio = 0; 0.004; 0.04; 0.25) were prepared by polymerizable complex method with the following reduction at 800 °C. A comparative study of the genesis, textural, structural and morphological properties of materials has been carried out by ex situ and in situ methods. It was shown that Ce0.75(NiCu)0.25O1.75 samples were fluorite-like ceria-based solid solutions with a mesoporous texture. Copper promotion has a positive effect on self-activation and hydrogen yield by improving the reducibility of the catalyst, lowering the exsolution temperature of the Ni2+ cations, and maintaining the concentration of oxygen vacancies. The catalyst of optimal composition NiCu/CeO2 (9.4 wt. % Ni, 0.4 wt. % Cu) formed from Ce0.75(NiCu)0.25O1.75 with Cu/Ni = 0.04 is resistant to coking and provides high hydrogen yield (82%) and CO2 utilization (78%) in steam/CO2 reforming of methane at 850 °C.
Graphical Abstract













Similar content being viewed by others
References
Global Hydrogen Review 2022 (2022) IEA. https://doi.org/10.1787/a15b8442-en. Accessed 25 April 2023
Ismagilov ZR, Matus EV, Li L (2022) Catalytic methods of converting carbon dioxide into useful products to reduce the impact of coal generation on global climate change. Phys Usp 65:1139–1154. https://doi.org/10.3367/UFNe.2021.07.039084
Energy Technology Perspectives (2020)—Special Report on Carbon Capture Utilisation and Storage (2020) IEA https://doi.org/10.1787/208b66f4-en.
Zhu Q (2019) Developments on CO2-utilization technologies. Clean Energy 3:85–100. https://doi.org/10.1093/ce/zkz008.Accessed25April2023
Quarton CJ, Samsatli S (2020) The value of hydrogen and carbon capture, storage and utilisation in decarbonising energy: insights from integrated value chain optimisation. Appl Energy 257:113936. https://doi.org/10.1016/j.apenergy.2019.113936
Yu M, Wang K, Vredenburg H (2021) Insights into low-carbon hydrogen production methods: green, blue and aqua hydrogen. Int J Hydrogen Energy 46:21261–21273. https://doi.org/10.1016/j.ijhydene.2021.04.016
Chen J, Han J, Xu D (2019) Efficient operation of autothermal microchannel reactors for the production of hydrogen by steam methane reforming. Int J Hydrogen Energy 44:11546–11563. https://doi.org/10.1016/j.ijhydene.2019.03.025
Cloete S, Khan MN, Amini S (2019) Economic assessment of membrane-assisted autothermal reforming for cost effective hydrogen production with CO2 capture. Int J Hydrogen Energy 44:3492–3510. https://doi.org/10.1016/j.ijhydene.2018.12.110
Rau F, Herrmann A, Krause H, Fino D, Trimis D (2019) Efficiency of a pilot-plant for the autothermal reforming of biogas. Int J Hydrogen Energy 4:19135–19140. https://doi.org/10.1016/j.ijhydene.2018.04.014
Ismagilov IZ, Vosmerikov AV, Korobitsyna LL, Matus EV, Kerzhentsev MA, Stepanov AA et al (2021) Promoters for improvement of the catalyst performance in methane valorization processes. Eurasian Chem J 23:147–168. https://doi.org/10.18321/ectj1099
Ismagilov ZR, Matus EV, Ismagilov IZ, Sukhova OB, Yashnik SA, Ushakov VA et al (2019) Hydrogen production through hydrocarbon fuel reforming processes over Ni based catalysts. Catal Today 323:166–182. https://doi.org/10.1016/j.cattod.2018.06.035
Yang E-H, Noh YS, Hong GH, Moon DJ (2018) Combined steam and CO2 reforming of methane over La1-xSrxNiO3 perovskite oxides. Catal Today 299:242–250. https://doi.org/10.1016/j.cattod.2017.03.050
Zhang Y, Wang J, Zhang G, Liu J, Dou L, Xu Y et al (2022) Combined steam and CO2 reforming of methane over Co–Ce/AC-N catalyst: effect of preparation methods on catalyst activity and stability. Int J Hydrogen Energy 47:2914–2925. https://doi.org/10.1016/j.ijhydene.2021.10.202
Lin YS, Tu JY, Tsai DH (2021) Steam-promoted methane-CO2 reforming by NiPdCeOx@SiO2 nanoparticle clusters for syngas production. Int J Hydrogen Energy 46:25103–25113. https://doi.org/10.1016/j.ijhydene.2021.05.053
Matus E, Sukhova O, Kerzhentsev M, Ismagilov I, Yashnik S, Ushakov V et al (2022) Hydrogen production through Bi-reforming of methane: improving Ni catalyst performance via an exsolution approach. Catalysts 12:1493. https://doi.org/10.3390/CATAL12121493
Matus EV, Sukhova OB, Ismagilov IZ, Ushakov VA, Yashnik SA, Kerzhentsev MA et al (2022) Steam/CO2 reforming of methane over impregnated Ni/CeO2 catalysts: effect of sample composition on their activity and stability. Eurasian Chem J 24:191–202. https://doi.org/10.18321/ectj1432
Paladino Lino AV, Rodella CB, Assaf EM, Assaf JM (2020) Methane tri-reforming for synthesis gas production using Ni/CeZrO2/MgAl2O4 catalysts: effect of Zr/Ce molar ratio. Int J Hydrogen Energy 45:8418–8432. https://doi.org/10.1016/j.ijhydene.2020.01.002
Wu KT, Yu CT, Chein RY (2017) Numerical modeling on catalytic tri-reforming reaction of methane for syngas production. Energy Procedia 105:4198–4203. https://doi.org/10.1016/j.egypro.2017.03.895
Kumar R, Pant KK (2020) Hydrotalcite-derived Ni–Zn–Mg–Al catalyst for Tri-reforming of methane: effect of divalent to trivalent metal ratio and Ni loading. Fuel Process Technol 210:106559. https://doi.org/10.1016/j.fuproc.2020.106559
Oni AO, Anaya K, Giwa T, Di Lullo G, Kumar A (2022) Comparative assessment of blue hydrogen from steam methane reforming, autothermal reforming, and natural gas decomposition technologies for natural gas-producing regions. Energy Convers Manag 254:115245. https://doi.org/10.1016/j.enconman.2022.115245
Johnson Matthey Group. LCHTM Process for the production of blue hydrogen 2022. https://matthey.com/documents/161599/474986/26367+JM+LCH+Process+-+Production+of+Low+Carbon+Hydrogen+TP+%28screen%29+13.pdf/71bb11a0-47ce-e609-412b-0678b1b4e5da?t=1654695668629. Accessed 25 April 2023
Smaller carbon footprint. Higher process efficiency. https://www.engineering.linde.com/dryref. Accessed 25 April 2023
de Medeiros FGM, Lopes FWB, Rego de Vasconcelos B (2022) Prospects and technical challenges in hydrogen production through dry reforming of methane. Catalysts 12:363. https://doi.org/10.3390/catal12040363
Wittich K, Krämer M, Bottke N, Schunk SA (2020) Catalytic dry reforming of methane: insights from model systems. ChemCatChem 12:2130–2147. https://doi.org/10.1002/cctc.201902142
Matus EV, Ismagilov ZR (2022) Promising directions in chemical processing of methane from coal industry. Part 1. Thermodynamic analysis. Eurasian Chem J 24:203–214. https://doi.org/10.18321/ectj1433
Johnson Matthey. Ammonia plant performance 2018. https://matthey.com/documents/161599/440146/JM+Ammonia+plant+performance+%28c2019%29.pdf/0dd39f7a-fbcc-7211-5230-c1d70678e6f1?t=1653488107209. Accessed 25 April 2023
Pinaeva LG, Noskov AS (2021) The modern level of catalysts and technologies for natural gas conversion to syngas. Katal v Promyshlennosti 21:308–330. https://doi.org/10.18412/1816-0387-2021-5-308-330
TOPSOE: Hydrogen. Reforming. https://www.topsoe.com/processes/hydrogen/reforming. Accessed 25 April 2023
Fowles M, Carlsson M (2021) Steam reforming of hydrocarbons for synthesis gas production. Top Catal 64:856–875. https://doi.org/10.1007/s11244-021-01496-z
Snoeck J-W, Froment GF, Fowles M (2002) Steam/CO2 reforming of methane. Carbon filament formation by the boudouard reaction and gasification by CO2, by H2, and by steam: kinetic study. Ind Eng Chem Res 41:4252–4265. https://doi.org/10.1021/ie010666h
Carlsson M (2015) Carbon formation in steam reforming and effect of potassium promotion. Johnson Matthey Technol Rev 59:313–318. https://doi.org/10.1595/205651315X688992
Sehested J, Gelten JAP, Helveg S (2006) Sintering of nickel catalysts: effects of time, atmosphere, temperature, nickel-carrier interactions, and dopants. Appl Catal A Gen 309:237–246. https://doi.org/10.1016/j.apcata.2006.05.017
Morales-Cano F, Lundegaard LF, Tiruvalam RR, Falsig H, Skjøth-Rasmussen MS (2015) Improving the sintering resistance of Ni/Al2O3 steam-reforming catalysts by promotion with noble metals. Appl Catal A Gen 498:117–125. https://doi.org/10.1016/j.apcata.2015.03.016
Cheephat C, Daorattanachai P, Devahastin S, Laosiripojana N (2018) Partial oxidation of methane over monometallic and bimetallic Ni-, Rh-, Re-based catalysts: effects of Re addition, co-fed reactants and catalyst support. Appl Catal A Gen 563:1–8. https://doi.org/10.1016/j.apcata.2018.06.032
Ismagilov IZ, Matus EV, Kuznetsov VV, Kerzhentsev MA, Yashnik SA, Prosvirin IP et al (2014) Hydrogen production by autothermal reforming of methane over NiPd catalysts: effect of support composition and preparation mode. Int J Hydrogen Energy 39:20992–21006. https://doi.org/10.1016/j.ijhydene.2014.10.044
Mohamedali M, Henni A, Ibrahim H (2018) Recent advances in supported metal catalysts for syngas production from methane. ChemEngineering 2:9. https://doi.org/10.3390/chemengineering2010009
Li D, Nakagawa Y, Tomishige K (2011) Methane reforming to synthesis gas over Ni catalysts modified with noble metals. Appl Catal A Gen 408:1–24. https://doi.org/10.1016/j.apcata.2011.09.018
Miyata T, Li D, Shiraga M, Shishido T, Oumi Y, Sano T et al (2006) Promoting effect of Rh, Pd and Pt noble metals to the Ni/Mg(Al)O catalysts for the DSS-like operation in CH4 steam reforming. Appl Catal A Gen 310:97–104. https://doi.org/10.1016/j.apcata.2006.05.022
Kerzhentsev MA, Matus EV, Rundau IA, Kuznetsov VV, Ismagilov IZ, Ushakov VA et al (2017) Development of a Ni–Pd/CeZrO2/Al2O3 catalyst for the effective conversion of methane into hydrogen-containing gas. Kinet Catal 58:601–622. https://doi.org/10.1134/S002315841705010X
Bengaard HS, Nørskov JK, Sehested J, Clausen BS, Nielsen LP, Molenbroek AM et al (2002) Steam reforming and graphite formation on Ni catalysts. J Catal 209:365–384. https://doi.org/10.1006/jcat.2002.3579
Rostrup-Nielsen JR, Sehested J, Noerskov JK (2003) Hydrogen and synthesis gas by steam- and CO2 reforming. Adv Catal 47:65–139. https://doi.org/10.1002/chin.200317288
Ismagilov IZ, Matus EV, Kuznetsov VV, Mota N, Navarro RM, Yashnik SA et al (2014) Hydrogen production by autothermal reforming of methane: effect of promoters (Pt, Pd, Re, Mo, Sn) on the performance of Ni/La2O3 catalysts. Appl Catal A Gen 481:104–115. https://doi.org/10.1016/j.apcata.2014.04.042
Yao Y, Goodman DW (2014) Direct evidence of hydrogen spillover from Ni to Cu on Ni–Cu bimetallic catalysts. J Mol Catal A Chem 283–284:239–242. https://doi.org/10.1016/j.molcata.2013.12.013
Bobadilla LF, Romero-Sarria F, Centeno MA, Odriozola JA (2016) Promoting effect of Sn on supported Ni catalyst during steam reforming of glycerol. Int J Hydrogen Energy 41:9234–9244. https://doi.org/10.1016/j.ijhydene.2016.04.119
Dedov AG, Loktev AS, Mukhin IE, Baranchikov AE, Ivanov VK, Bykov MA et al (2019) Effect of the support nature on stability of nickel and nickel–cobalt catalysts for partial oxidation and dry reforming of methane to synthesis gas. Pet Chem 59:385–393. https://doi.org/10.1134/S0965544119040042
Chatla A, Ghouri MM, El Hassan OW, Mohamed N, Prakash AV, Elbashir NO (2020) An experimental and first principles DFT investigation on the effect of Cu addition to Ni/Al2O3 catalyst for the dry reforming of methane. Appl Catal A Gen 602:117699. https://doi.org/10.1016/j.apcata.2020.117699
Nataj SMM, Alavi SM, Mazloom G (2018) Modeling and optimization of methane dry reforming over Ni–Cu/Al2O3 catalyst using Box-Behnken design. J Energy Chem 27:1475–1488. https://doi.org/10.1016/j.jechem.2017.10.002
Matus EV, Shlyakhtina AS, Sukhova OB, Ismagilov IZ, Ushakov VA, Yashnik SA et al (2019) Effect of preparation methods on the physicochemical and functional properties of Ni/CeO2 catalysts. Kinet Catal 60:221–230. https://doi.org/10.1134/S002315841902006X
Kerzhentsev MA, Matus EV, Ismagilov IZ, Ushakov VA, Stonkus OA, Larina TV et al (2017) Structural and morphological properties of Ce1–xMxOy (M = Gd, La, Mg) supports for the catalysts of autothermal ethanol conversion. J Struct Chem 58:126–134. https://doi.org/10.1134/S002247661701019X
Melchionna M, Trovarelli A, Fornasiero P (2020) Synthesis and properties of cerium oxide-based materials. Elsevier Inc. 13–43 https://doi.org/10.1016/b978-0-12-815661-2.00002-5.
Montini T, Melchionna M, Monai M, Fornasiero P (2016) Fundamentals and catalytic applications of CeO2-based materials. Chem Rev 116:5987–6041. https://doi.org/10.1021/acs.chemrev.5b00603
Guo M, Lu J, Wu Y, Wang Y, Luo M (2011) UV and visible Raman studies of oxygen vacancies in rare-earth-doped ceria. Langmuir 27:3872–3877. https://doi.org/10.1021/la200292f
Lever ABP (1984) Inorganic Electron Spectroscopy. second ed. Elsevier, Amsterdam – Oxford – New York – Tokyo.
Wu L, Wiesmann HJ, Moodenbaugh AR, Klie RF, Zhu Y, Welch DO et al (2004) Oxidation state and lattice expansion of CeO2−x nanoparticles as a function of particle size. Phys Rev B 69:125415. https://doi.org/10.1103/PhysRevB.69.125415
Marinho ALA, Rabelo-Neto RC, Epron F, Bion N, Toniolo FS, Noronha FB (2020) Embedded Ni nanoparticles in CeZrO2 as stable catalyst for dry reforming of methane. Appl Catal B Environ 268:118387. https://doi.org/10.1016/j.apcatb.2019.118387
Fuentes RO, Acuña LM, Albornoz CA, Leyva AG, Sousa N, Figueiredo FM (2016) Structural, physical and chemical properties of nanostructured nickel-substituted ceria oxides under reducing and oxidizing conditions. RSC Adv 6:64861–64870. https://doi.org/10.1039/c6ra14853k
Pinto FM, Suzuki VY, Silva RC, La Porta FA (2019) Oxygen defects and surface chemistry of reducible oxides. Front Mater 6:260. https://doi.org/10.3389/fmats.2019.00260
Koeppel RA, Baiker A, Wokaun A (1992) Copper/zirconia catalysts for the synthesis of methanol from carbon dioxide. Appl Catal A Gen 84:77–102. https://doi.org/10.1016/0926-860X(92)80340-I
Dumas JM, Geron C, Kribii A, Barbier J (1989) Preparation of supported copper catalysts. Appl Catal 47:L9-15. https://doi.org/10.1016/S0166-9834(00)83256-X
Hu Y, Dong L, Shen M, Liu D, Wang J, Ding W et al (2001) Influence of supports on the activities of copper oxide species in the low-temperature NO+CO reaction. Appl Catal B Environ 31:61–69. https://doi.org/10.1016/S0926-3373(00)00269-1
Moretti E, Lenarda M, Storaro L, Talon A, Frattini R, Polizzi S et al (2007) Catalytic purification of hydrogen streams by PROX on Cu supported on an organized mesoporous ceria-modified alumina. Appl Catal B Environ 72:149–156. https://doi.org/10.1016/j.apcatb.2006.10.020
Yu Q, Liu L, Dong L, Li D, Liu B, Gao F et al (2010) Effects of Ce/Zr ratio on the reducibility, adsorption and catalytic activity of CuO/CexZr1-xO2/γ-Al2O3 catalysts for NO reduction by CO. Appl Catal B Environ 96:350–360. https://doi.org/10.1016/j.apcatb.2010.02.032
Rodriguez JA, Kim JY, Hanson JC, Pérez M, Frenkel AI (2003) Reduction of CuO in H2: in situ time-resolved XRD studies. Catal Lett 85:247–254. https://doi.org/10.1023/A:1022110200942
Lamonier C, Ponchel A, D’Huysser A, Jalowiecki-Duhamel L (1999) Studies of the cerium-metal-oxygen-hydrogen system (metal = Cu, Ni). Catal Today 50:247–259. https://doi.org/10.1016/S0920-5861(98)00507-0
Shan W, Luo M, Ying P, Shen W, Li C (2003) Reduction property and catalytic activity of Ce1−xNixO2 mixed oxide catalysts for CH4 oxidation. Appl Catal A Gen 246:1–9. https://doi.org/10.1016/S0926-860X(02)00659-2
Vita A, Italiano C, Fabiano C, Laganà M, Pino L (2015) Influence of Ce-precursor and fuel on structure and catalytic activity of combustion synthesized Ni/CeO2 catalysts for biogas oxidative steam reforming. Mater Chem Phys 163:337–347. https://doi.org/10.1016/j.matchemphys.2015.07.048
Shen H, Li H, Yang Z, Li C (2022) Magic of hydrogen spillover: understanding and application. Green Energy Environ 7:1161–1198. https://doi.org/10.1016/j.gee.2022.01.013
Acknowledgements
This work was supported by the Ministry of Science and Higher Education of the Russian Federation within the governmental order for the Boreskov Institute of Catalysis. The XRD, UV–Vis DRS and TEM studies were carried out using facilities of the shared research center “National center of investigation of catalysts” at the Boreskov Institute of Catalysis. The authors also acknowledge the resource center “VTAN” (Novosibirsk State University) for the access to TEM equipment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Notes
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Matus, E.V., Sukhova, O.B., Kerzhentsev, M.A. et al. Hydrogen Production from Methane with CO2 Utilization over Exsolution Derived Bimetallic NiCu/CeO2 Catalysts. Catal Lett 154, 2197–2210 (2024). https://doi.org/10.1007/s10562-023-04454-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-023-04454-4