Abstract
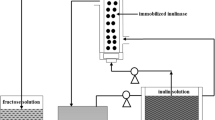
The objective of this study was to convert inulin to high output fructose with the commercial inulinase in the continuous ultrafiltration membrane bioreactor (UFMB). The effects of operational conditions were explored for the volumetric productivity. The model equations of kinetics and membrane fouling were also founded. The stable fructose production with the volumetric productivity was attained at the optimum condition of inulin concentration (100 g/L), E/S ratio (40 U/g) and residence time (20 min). The kinetics results showed that the experimental data obtained were fit to the model equations. Preliminary experimental results were attained the fundamental membrane fouling mechanism. These results demonstrated its feasibility and availability for efficient fructose production, which were beneficial to provide theoretical basis for further researches.
Graphic Abstract
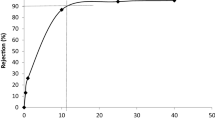
The operational conditions were explored for the volumetric productivity. Model equations and parameters of kinetics were founded and obtained. The membrane fouling mechanism of UFMB system was founded.











Similar content being viewed by others
References
Singh RS, Dhaliwal R, Puri M (2007) Partial purification and characterization of exoinulinase from Kluyveromyces marxianus YS-1 for preparation of high-fructose syrup. J Ind Microbiol Biotechn 34:649–655
Silva MF, Rigo D, Mossi V et al (2013) Evaluation of enzymatic activity of commercial inulinase from Aspergillus niger immobilized in polyurethane foam. Food Bioprod Proc 91:54–59
Chi ZM, Zhang T, Cao TS et al (2011) Biotechnological potential of inulin for bioprocesses. Biores Techn 102:4295–4303
Rocha JR, Catana R, Ferreira BS et al (2006) Design and characterisation of an enzyme system for inulin hydrolysis. Food Chem 95:77–82
Award GEA, Wehaidy HR, Abd EAAA et al (2017) A novel alginate-CMC gel beads for efficient covalent inulinase immobilization. Colloid Polym Sci 295:495–506
Singh RS, Chauhan K, Pandey A et al (2018) Biocatalytic strategies for the production of high fructose syrup from inulin. Biores Techn 260:395–403
Hang H, Cheng X, Yan F et al (2020) Immobilized inulinase for the continuous conversion of inulin in the fluidized-bed reactor. Catal Lett 150:1849–1855
Chiang WD, Shih CJ, Chu YH (1999) Functional properties of soy protein hydrolysate produced from a continuous membrane reactor system. Food Chem 65:189–194
Hang H, Mu W, Jiang B et al (2012) Enzymatic hydrolysis of inulin in a bioreactor coupled with an ultrafiltration membrane. Desalination 284:309–315
Merz M, Eisele T, Claaben W et al (2015) Continuous long-term hydrolysis of wheat gluten using a principally food-grade enzyme membrane reactor system. Biochem Eng J 99:14–123
Stefania B, Neha B, Sandra VR et al (2017) Continuous production of pectic oligosaccharides from onion skins with an enzyme membrane reactor. Food Chem 267:101–110
Paolucci-Jeanjean D, Belleville MP, Rios GM et al (2000) Kinetics of continuous starch hydrolysis in a membrane reactor. Biochem Eng J 6:233–238
Mazzei R, Giorno L, Piacentini E et al (2009) Kinetic study of a biocatalytic membrane reactor containing immobilized β-glucosidase for the hydrolysis of oleuropein. J Membr Sci 339:215–233
Bhatia S, Long WS, Kamaruddin AH (2004) Enzymatic membrane reactor for the kinetic resolution of racemic ibuprofen ester: modeling and experimental studies. Chem Eng Sci 59:5061–5068
Zhao L, Qiao J, Moon MH et al (2018) Construction of a thermoresponsive magnetic porous polymer membrane enzyme reactor for glutaminase kinetics study. Analyt Bioanalyt Chem 410:5211–5218
Vasan SS, Field RW (2006) On maintaining consistency between the film model and the profile of the concentration polarization layer. J Membr Sci 279:434–438
Bódalo A, Gómez JL, Gómez E et al (2001) Ultrafiltration membrane reactors for enzymatic resolution of aminoacids: design model and optimization. Enzym Microbiol Techn 28:355–361
Lowry O, Rosebrough N, Farr A et al (1951) Protein measurement with the folin-phenol reagent. J Biol Chem 31:426–428
Hang H, Wang C, Cheng Y et al (2018) Design and properties of an immobilization enzyme system for inulin conversion. Appl Biochem Biotechn 184:453–470
Sims KA, Cheryan M (1992) Hydrolysis of liquefied corn starch in a membrane reactor. J Biotechn Bioeng 39:960–967
Hermans PH, Bredee HL (1936) Principles of the mathematical treatment of constant pressure filtration. J Soc Chem Ind 55:1–4
Acknowledgements
This work was supported by the Fundamental Research Funds for 2019 Project of Anhui Natural Science Foundation (Grant No. 1908085MC99) and 2016 Key Projects of Anhui Educational Department (Grant No.611607) and 2012-year Dr. Activation Fee for Scientific Research Project (Grant No. 161-070110). The authors are grateful to Anhui Natural Science Foundation, College of Life Sciences of AHNU, for giving necessary funding and facility to carry out the work.
Author information
Authors and Affiliations
Contributions
All the six authors have contributed equally for the research work. They are all the team players of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
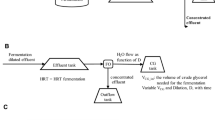
was defined as the total mass of fructose (g) gained in all permeates gathered up to time t; t was equal to the collection time (min); 0.43 L was determined as the total volume of the reaction mixture.
Slo substrate concentration (g/L) in the inlet feed, Sl substrate concentration (g/L) in the outlet stream, (-v) rate of substrate depletion, F flow rate (mL/min) of the feed and the filtrate, VR bioreactor volume (mL).
\(k2\) kinetic constant (Vmax = k2Sl0E), Kis inhibition constant for substrate, P production concentration, Keq equilibrium constant, Km Michaelis constant.
Lp and ΔP are defined as the parameters of the linear coefficient and transmembrane pressure, respectively.
J (L/h/m2) is permeate flux, ΔP (bar) is transmembrane pressure, \(\mu \left( {{\text{P}}_{{\text{a}}} \cdot {\text{s}}} \right)\) is liquor viscosity and R (1/m) is hydraulic resistance.
Rm is membrane resistance that reckoned on regarding the pure water permeability of unused UF membrane; Rr is reversible fouling resistance, Ri is irreversible fouling resistance that calculated regarding the pure water permeability after rinsing steps. The overall fouling resistance (Rf) is given by the sum of Rr and Ri.
ΔM is the permeate gathered during the period of time (Δt), \(\rho\) is the solution density and A is the filtration area of UF membrane.
Rights and permissions
About this article
Cite this article
Hua, H., Changbao, W., Liuli, S. et al. Characters of Inulin Conversion Induced by Inulinase in Ultrafiltration Membrane Bioreactor. Catal Lett 152, 1692–1702 (2022). https://doi.org/10.1007/s10562-021-03761-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-021-03761-y