Abstract
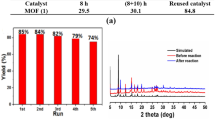
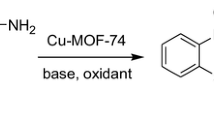
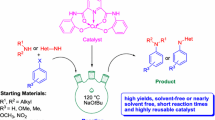
The copper benzene-1, 3, 5-tricarboxylate metal–organic framework (CuBTC) was found to be an effective heterogeneous catalyst for the aza-Michael addition reaction of the four types of amines to electron deficient alkenes at room temperature. The catalytic protocol showed high product yields and outstanding chemo selectivity. The cyclic amines (piperidine and pyrrolidine) and aliphatic amines (n-dibutylamine) provided aza-Michael addition with a high yield of product (⁓98%) within shorter reaction period (2 h) at room temperature under mild reaction conditions using CuBTC. However, it was observed that the aza-Michael reaction proceeded more slowly, giving 62% yield of product after 24 h in the case of aromatic amine (aniline) with n-butyl acrylate in the presence of CuBTC under identical reaction conditions. The catalyst could be reused four recycles without losing its initial catalytic activity and selectivity. XRD and SEM analysis further confirmed that the crystallinity of catalyst was retained during the reaction. A reaction mechanism is proposed for the aza-Michael addition reaction over heterogeneous CuBTC catalyst.
Graphic Abstract











Similar content being viewed by others
References
Rulev AY (2011) Aza-Michael reaction: achievements and prospects. Russ Chem Rev 80:197–218
Sánchez-Rosellό M, Aceña JL, Simόn-Fuentes A, Pozo del CA (2014) A general overview of the organocatalytic intramolecular aza-Michael reaction. Chem Soc Rev 43:7430–7453
Cabral J, Laszlo P, Mahe L, Montaufier MT, Randriamahefa SL (1989) Catalysis of the specific michael addition: The example of acrylate acceptors. Tetrahedron Lett 30:3969–3972
Genest D, Portinha D, Fleury E, Ganachaud F (2017) The aza-Michael reaction as an alternative strategy to generate advanced silicon-based (macro) molecules and materials. Prog Poly Sci 72:61–110
Xu LW, Li L, **a CG (2004) Transition-Metal-Based Lewis Acid Catalysis of Aza-Type Michael Additions of Amines to α, β-Unsaturated Electrophiles in Water. Helv Chim Acta 87:1522–1526
Azizi N, Saidi MR (2004) LiClO4 Accelerated Michael addition of amines to α, β-unsaturated olefins under solvent-free conditions. Tetrahedron 60:383–387
Xu LW, Li JW, **a CG, Zhou SL, Hu XX (2003) Efficient Copper-Catalyzed Chemo Selective Conjugate Addition of Aliphatic Amines to α, β-Unsaturated Compounds in Water. Synlett 15:2425–2427
Bartoli G, Bosco M, Marcantoni E, Pertrini M, Sambri L, Torregiani E (2001) Conjugate Addition of Amines to α, β-Enones Promoted by CeCl3·7H2O−NaI System Supported in Silica Gel. J Org Chem 66:9052–9055
Srivastava N, Banik BK (2003) Bismuth Nitrate-Catalyzed Versatile Michael Reaction. J Org Chem 68:2109–2114
Giovanna B, Roderick A (2016) Aza-Michael Mono-addition Using Acidic Alumina under Solventless Conditions. Molecules 21:815
Kantam ML, Neelima B, Reddy CV, Chakravarti R (2007) Aza-Michael Addition of Imidazoles to α, β-Unsaturated Compounds and Synthesis of β-Amino Alcohols via Nucleophilic Ring Opening of Epoxides Using Copper(II) Acetylacetonate (Cu(acac)2) Immobilized in Ionic Liquids. Ind Eng Chem Res 46:8614–8619
Ying A, Wang L, Deng H, Chen J, Chen X, Ye W (2009) Aza-Michael addition of aliphatic or aromatic amines to α, β-unsaturated compounds catalyzed by a DBU-derived ionic liquid under solvent-free conditions. Tetrahedron Lett 50:1653–1657
Ghasemi MH, Kowsari E, Shafiee A (2016) Aza-Michael-type addition reaction catalysed by a supported ionic liquid phase incorporating an anionic heteropoly acid. Tetrahedron Lett 57:1150–1153
Kumar S, Kaur A, Singh V (2019) Efficient protocol for Aza-Michael addition of N-heterocycles to α, β-unsaturated compound using [Ch]OH and [n-butyl urotropinium] OH as basic ionic liquids in aqueous/solvent free conditions. Synth Commun 49:193–201
Boruah K, Borah R (2019) Design of water stable 1,3-Dialkyl- 2-methyl imidazolium basic ionic liquids as reusable homogeneous catalysts for aza-michael reaction in neat condition. ChemistrySelect 4:3479–3485
Kantam ML, Neelima B, Reddy CV (2005) A recyclable protocol for aza-Michael addition of amines to α, β-unsaturated compounds using Cu-Al hydrotalcite. J Mol Catal A: Chem 241:147–150
Esteves PA, Esloa M, Rodriguez LM, Olivia-compost A, Radim H (2007) Aza-Michael reactions with vinyl sulfones and Amberlyst-15 as catalyst. Tetrahedron Lett 48:9040–9043
Nath J, Chaudhuri MK (2009) Phosphate Impregnated Titania: An Efficient Reusable Heterogeneous Catalyst for Aza-Michael Reactions under Solvent-Free Condition. Catal Lett 133:388–393
Saidi MR, Pourshojaei Y, Aryanasa F (2009) Highly Efficient Michael Addition Reaction of Amines Catalyzed by Silica-Supported Aluminum Chloride. Synth Commun 39:1109–1119
Bosica G, Spiteri J, Borg C (2014) Aza-Michael reaction: selective mono- versus bis-addition under environmentally-friendly conditions. Tetrahedron 70:2449–2454
Kalita P, Pegu CD, Dutta P, Baruah PK (2014) Room temperature solvent free aza-Michael reactions over nano-cage mesoporous materialsJ. Mol Catal A 394:145–150
Hosseinzadeh R, Aghili N (2016) M. Synthesis, Characterization and Catalytic Application of MCM-41 Supported Phenanthrolinium Dibromide Catalyst for Aza Michael Addition Reaction in Aqueous Medium. Catal Lett 146:1194–1203
Rathod PB, Kumar KSA, Athawale AA, Pandey AKS, Chattopadhyay S (2018) Polymer-Shell-Encapsulated Magnetite Nanoparticles Bearing Hexamethylenetetramine for Catalysing Aza-Michael Addition Reactions. Eur J Org Chem 43:5980–5987
Hakiki A, Kerbadou RM, Boukoussa B, Zahmani HH, Launay F, Pailleret A, Pillier F, Hacini S, Bengueddach A, Hamacha RJ (2019) Inorg Organomet Poly Mater 29:1773–1784
Corma A, Garcia H, LlabrésiXamena FX (2010) Engineering Metal Organic Frameworks for Heterogeneous Catalysis. Chem Rev 110:4606–4655
Bhattacharjee S, Lee YR, Puthiaraj P, Cho SM, Ahn WS (2015) Metal-Organic Frameworks for Catalysis. Catal Surv Asia 19:203–222
Hu ML, Safarifard V, Doustkhah E, Rostamnia S, Morsali A, Nouruzi N, Beheshti S, Akhbari K (2018) Taking organic reactions over metal-organic frameworks as heterogeneous catalysis. Micropor Mesopor Mater 256:111–127
Rostamnia S, Mohsenzad F (2018) Nanoarchitecturing of open metal site Cr-MOFs for oxodiperoxo molybdenum complexes [MoO(O2)2@En/MIL-100(Cr)] as promising and bifunctional catalyst for selective thioether oxidation. Mol Catal 445:12–20
Rostamnia S, Alamgholiloo H, Jafari M (2018) Ethylene diamine post-synthesis modification on open metal site Cr-MOF to access efficient bifunctional catalyst for the Hantzsch condensation reaction. Appl Organomet Chem 32:e4370
Alamgholiloo H, Zhang S, Ahadi A, Rostamnia S, Banaei R, Li Z, Liu X, Shokouhimehr M (2019) Synthesis of bimetallic 4-PySI-Pd@Cu(BDC) via open metal site Cu-MOF: Effect of metal and support of Pd@Cu-MOFs in H2 generation from formic acid. Mol Catal 467:30–37
Panahi P, Nouruzi N, Doustkhah E, Mohtasham H, Ahadi A, Ghiasi-Moaser A, Rostamnia S, Mahmoudi G, Khataeed A (2019) Zirconium based porous coordination polymer (PCP) bearing organocatalytic ligand: A promising dual catalytic center for ultrasonic heterocycle synthesis. Ultrason Sonchem 58:104653
Alamgholiloo H, Rostamnia S, Zhang K, Lee TH, Lee YS, Varma RS, Jang HW, Shokouhimehr M (2020) Boosting Aerobic Oxidation of Alcohols via Synergistic Effect between TEMPO and a Composite Fe3O4/Cu-BDC/GO Nanocatalyst. ACS Omega 5:5182–5191
Alamgholiloo H, Rostamnia S, Hassankhani A, Liu X, Eftekhari A, Hasanzadeh A, Zhang K, Karimi-Maleh H, Khaksar S, Varma RS, Shokouhimehr M (2020) Formation and stabilization of colloidal ultra-small palladium nanoparticles on diamine-modified Cr-IL-101: Synergic boost to hydrogen production from formic acid. J Colloid Interf Sci 567:126–135
Jang MS, Yu K, Lee J, Ahn WS (2020) Sonochemical synthesis of rho-ZMOF catalyst for an enhanced CO2 cycloaddition reaction. Mater Lett 277:128387
Kim J, Cho HY, Ahn WS (2012) Synthesis and Adsorption/Catalytic Properties of the Metal Organic Framework CuBTC. Catal Surv Asia 16:106–119
Hall J, Bollini P (2019) Structure, characterization, and catalytic properties of open-metal sites in metal organic frameworks. React Chem Eng 4:207–222
Kökçam-Demir U, Goldman A, Esrafili L, Gharib M, Morsali A, Weingart O, Janiak C (2020) Coordinatively Unsaturated Metal Sites (Open Metal Sites) in Metal-Organic Frameworks: Design and Applications. Chem Soc Rev 49:2751–2798
Rostamnia S, Alamgholiloo H (2018) Synthesis and Catalytic Application of Mixed Valence Iron (FeII/FeIII)-Based OMS-MIL-100(Fe) as an Efficient Green Catalyst for the azaMichael Reaction. Catal Lett 148:2918–2928
Savonnet M, Aguado S, Ravon U, Bazer-Bachi D, Lecocq V, Bats N, Pinel C, Farrusseng D (2009) Solvent free base catalysis and transesterification over basic functionalised Metal-Organic Frameworks. Green Chem 11:1729–1732
Nguyen LTL, Nguyen TT, Nguyen KD, Phan NTS (2012) Metal–organic framework MOF-199 as an efficient heterogeneous catalyst for the aza-Michael reaction. Appl Catal A Gen 425–426:44–52
Kim J, Kim SH, Yang ST, Yang ST, Ahn WS (2012) Bench-scale preparation of Cu3(BTC)2 by ethanol reflux: Synthesis optimization and adsorption/catalytic applications. Micropor Mesopor Mater 161:48–55
Douraki SM, Massah AR (2015) The zeolite ZSM-5-SO3H catalyzed aza-Michael addition of amines and sulfonamides to electron-deficient alkenes under solvent-free conditions. Indian J Chem 54B:1346–1349
Dai L, Zhang Y, Dou Q, Wang X, Chen Y (2013) Chemo/regioselective Aza-Michael additions of amines to conjugate alkenes catalyzed by polystyrene-supported AlCl3. Tetrahedron 69:1712–1716
Acknowledgements
This work was supported by University of Dhaka (CARS) internal fund and in part by the Basic Science Research Program through the National Research Foundation of Korea (NRF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhattacharjee, S., Shaikh, A.A. & Ahn, WS. Heterogeneous Aza-Michael Addition Reaction by the Copper-Based Metal–Organic Framework (CuBTC). Catal Lett 151, 2011–2018 (2021). https://doi.org/10.1007/s10562-020-03459-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03459-7