Abstract
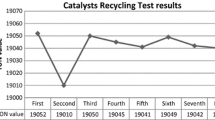
Ethylene glycol reduction method was used to prepare alumina supported Ru nanoparticles with different concentrations. All the synthesized materials were examined by different analytical techniques like XRD, TEM, EDX, H2-chemisorption, XPS and H2-TPD analysis. The performance of all the well synthesized Ru@Al2O3-x (x = 2–10 Ru wt%) catalysts were tested for CO2 hydrogenation reaction with or without ionic liquid medium. The influence of the physiochemical properties of Ru@Al2O3-x (x = 2–10 Ru wt%) catalysts was clearly observed during the catalysis CO2 hydrogenation reaction. The maximum catalytic activity was recorded with Ru@Al2O3-2 catalyst in [DAMI][CF3CF2CF2CF2SO3] ionic liquid. In this system, ionic liquid was noted as catalyst stabilizer and shifted the chemical equilibrium of CO2 hydrogenation reaction towards formic acid synthesis followed by the formation of intermediate carbonate. The Ru@Al2O3-2 catalyst in [DAMI][CF3CF2CF2CF2SO3] ionic liquid gave the best result in terms of formic acid synthesis and catalyst recycling (8 runs).
Graphical Abstract










Similar content being viewed by others
References
Dhakshinamoorthy A, Navalon S, Alvaro M, Garcia H (2012) Metal nanoparticles as heterogeneous fenton catalysts. Chemsuschem 5:46–64
Kalidindi SB, Jagirdar BR (2012) Nanocatalysis and prospects of green chemistry. Chemsuschem 5:65–75
Fan J, Gao Y (2006) Nanoparticle-supported catalysts and catalytic reactions—a mini-review. J Exp Nanosci 1:457–475
Munnik P, de Jongh PE, de Jong KP (2015) Recent developments in the synthesis of supported catalysts. Chem Rev 115:6687–6718
Lambert S, Cellier C, Grange P et al (2004) Synthesis of Pd/SiO2, Ag/SiO2, and Cu/SiO2 cogelled xerogel catalysts: study of metal dispersion and catalytic activity. J Catal 221:335–346
Mokrane T, Boudjahem A-G, Bettahar M (2016) Benzene hydrogenation over alumina-supported nickel nanoparticles prepared by polyol method. RSC Adv 6:59858–59864
Boudjahem A-G, Redjel A, Mokrane T (2012) Preparation, characterization and performance of Pd/SiO2 catalyst for benzene catalytic hydrogenation. J Ind Eng Chem 18(303–308):8
Hu H, **n JH, Hu H et al (2015) Synthesis and stabilization of metal nanocatalysts for reduction reactions—a review. J Mater Chem A 3:11157–11182
Liu L, Corma A (2018) Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem Rev 118:4981–5079
Boudjahem A-G, Mokrane T, Redjel A, Bettahar MM (2010) Silica supported nanopalladium prepared by hydrazine reduction. Compt Rendus Chim 13:1433–1439
Penzien J, Haeßner C, Jentys A et al (2004) Heterogeneous catalysts for hydroamination reactions: structure–activity relationship. J Catal 221:302–312
Li Y, Bastakoti BP, Abe H et al (2015) A dual soft-template synthesis of hollow mesoporous silica spheres decorated with Pt nanoparticles as a CO oxidation catalyst. RSC Adv 5:97928–97933
Panpranot J, Tangjitwattakorn O, Praserthdam P, Goodwin JG (2005) Effects of Pd precursors on the catalytic activity and deactivation of silica-supported Pd catalysts in liquid phase hydrogenation. Appl Catal A Gen 292:322–327
Gaillard F, Li X, Uray M, Vernoux P (2004) Electrochemical promotion of propene combustion in air excess on perovskite catalyst. Catal Lett 96:177–183
Nagai M, Huang J, Cui D et al (2017) Two-step reprecipitation method with size and zeta potential controllability for synthesizing semiconducting polymer nanoparticles. Colloid Polym Sci 295:1153–1164
Renna LA, Boyle CJ, Gehan TS, Venkataraman D (2015) Polymer nanoparticle assemblies: a versatile route to functional mesostructures. Macromolecules 48:6353–6368
Wang Z, Huang J, Huang W et al (2019) Agglomeration controllable reprecipitation method using solvent mixture for synthesizing conductive polymer nanoparticles. Colloid Polym Sci 297:69–76
Potai R, Traiphol R (2013) Controlling chain organization and photophysical properties of conjugated polymer nanoparticles prepared by reprecipitation method: the effect of initial solvent. J Colloid Interface Sci 403:58–66
Shenhar R, Norsten TB, Rotello VM (2005) Polymer-mediated nanoparticle assembly: structural control and applications. Adv Mater 17:657–669
Jeevanandam J, Barhoum A, Chan YC, Dufresne A, Danquah MK (2018) Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol 9:1050–1074
Gawande MB, Zboril R, Malgras V, Yamauchi Y (2015) Integrated nanocatalysts: a unique class of heterogeneous catalysts. J Mater Chem A 3:8241–8245
Polshettiwar V, Varma RS (2010) Green chemistry by nano-catalysis. Green Chem 12:743–754
Mondal J, Biswas A, Chiba S, Zhao Y (2015) Cu0 nanoparticles deposited on nanoporous polymers: a recyclable heterogeneous nanocatalyst for Ullmann coupling of aryl halides with amines in water. Sci Rep 5:8294
Chaturvedi S, Dave PN, Shah NK (2012) Applications of nano-catalyst in new era. J Saudi Chem Soc 16:307–325
Jiang H-L, Xu Q (2011) Porous metal–organic frameworks as platforms for functional applications. Chem Commun 47:3351–3370
Mohamedali M, Henni A, Ibrahim H (2018) Recent advances in supported metal catalysts for syngas production from methane. ChemEngineering 2:1–23
Trueba M, Trasatti SP (2005) γ-Alumina as a support for catalysts: a review of fundamental aspects. Eur J Inorg Chem 17:3393–3403
Singh V, Sapehiyia V, Srivastava V, Kaur S (2006) ZrO2-pillared clay: an efficient catalyst for solventless synthesis of biologically active multifunctional dihydropyrimidinones. Catal Commun 7:571–578
Upadhyay PR, Srivastava V (2016) Silica tethered ruthenium catalyst for the hydrogenation of CO2 gas. Lett Org Chem 13:380–387
Upadhyay PR, Srivastava V (2016) Heterogeneous silica tethered ruthenium catalysts for carbon sequestration reaction. Catal Lett 146:1478–1486
Upadhyay PR, Srivastava V (2016) Titanium dioxide supported ruthenium nanoparticles for carbon sequestration reaction. Nanosystems 7:513–517
Ghiaci M, Valikhani D, Sadeghi Z (2012) Synthesis and characterization of silica-supported Pd nanoparticles and its application in the Heck reaction. Chin Chem Lett 23:887–890
Srivastava V (2017) Mesoporous silica supported Ru nanoparticles for hydrogenation of CO2 molecule. Lett Org Chem 14:74–79
Srivastava V (2017) Active ruthenium(0) nanoparticles catalyzed wittig-type olefination reaction. Catal Lett 147:693–703
Srivastava V (2016) Active heterogeneous Ru nanocatalysts for CO2 hydrogenation reaction. Catal Letters 146:2630–2640
Srivastava V (2014) Ru-exchanged MMT clay with functionalized ionic liquid for selective hydrogenation of CO2 to formic acid. Catal Lett 144:2221–2226
Balu AM, Pineda A, Campelo JM, Gai PL, Luque R, Romero AA (2010) Fe/Al synergy in Fe2O3 nanoparticles supported on porous aluminosilicate materials: excelling activities in oxidation reactions. Chem Commun 46:7825–7827
Upadhyay P, Srivastava V (2016) Synthesis of monometallic Ru/TiO2 catalysts and selective hydrogenation of CO2 to formic acid in ionic liquid. Catal Letters 146:12–21
Ramprakash Upadhyay P, Srivastava V (2016) Selective hydrogenation of CO to methane over TiO2-supported ruthenium nanoparticles. In: Proceedings on materials today. pp 4093–4096
Upadhyay PR, Srivastava V (2016) Selective hydrogenation of CO2 gas to formic acid over nanostructured Ru-TiO2 catalysts. RSC Adv 6:42297–42306
Bulushev DA, Ross JRH (2018) Towards sustainable production of formic acid. Chemsuschem 11:821–836
Reymond H, Corral-Pérez JJ, Urakawa A, Rudolf von Rohr P (2018) Towards a continuous formic acid synthesis: a two-step carbon dioxide hydrogenation in flow. React Chem Eng 3:912–919
Bulushev DA, Ross JRH (2018) Heterogeneous catalysts for hydrogenation of CO2 and bicarbonates to formic acid and formates. Catal Rev 60:566–593
van Putten R, Wissink T, Swinkels T, Pidko EA (2019) Fuelling the hydrogen economy: scale-up of an integrated formic acid-to-power system. Int J Hydr Energy 56(26):7531–7534
Mellmann D, Sponholz P, Junge H, Beller M (2016) Formic acid as a hydrogen storage material—development of homogeneous catalysts for selective hydrogen release. Chem Soc Rev 45:3954–3988
Álvarez A, Bansode A, Urakawa A et al (2017) Challenges in the greener production of formates/formic acid, methanol, and dme by heterogeneously catalyzed CO2 hydrogenation processes. Chem Rev 117:9804–9838
Weilhard A, Qadir MI, Sans V, Dupont J (2018) Selective CO2 hydrogenation to formic acid with multifunctional ionic liquids. ACS Catal 8:1628–1634
Saeidi S, Amin S, Rahimpour MR (2014) Hydrogenation of CO2 to value-added products—a review and potential future developments. J CO2 Util 5:66–81
Upadhyay P, Srivastava V (2015) Ruthenium nanoparticle-intercalated montmorillonite clay for solvent-free alkene hydrogenation reaction. RSC Adv 5:740–745
Upadhyay PR, Srivastava V (2017) Ionic liquid mediated in situ synthesis of Ru nanoparticles for CO2 hydrogenation reaction. Catal Letters 147:1051–1060
Srivastava VK, Eilbracht P (2009) Ruthenium carbonyl-complex catalyzed hydroaminomethylation of olefins with carbon dioxide and amines. Catal Commun 10:1791–1795
Brennecke JF, Gurkan BE (2010) Ionic liquids for CO2 capture and emission reduction. J Phys Chem Lett 1:3459–3464
Bates ED, Mayton RD, Ntai I, Davis JH (2002) CO2 capture by a task-specific ionic liquid. J Am Chem Soc 124:926–927
Dai W-L, Luo S-L, Yin S-F, Au C-T (2009) The direct transformation of carbon dioxide to organic carbonates over heterogeneous catalysts. Appl Catal A Gen 366:2–12
Srivastava V (2014) In situ generation of Ru nanoparticles to catalyze CO2 hydrogenation to formic acid. Catal Letters 144:1745–1750
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gautam, P., Upadhyay, P.R. & Srivastava, V. Selective Hydrogenation of CO2 to Formic Acid over Alumina-Supported Ru Nanoparticles with Multifunctional Ionic Liquid. Catal Lett 149, 1464–1475 (2019). https://doi.org/10.1007/s10562-019-02773-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02773-z