Abstract
The mechanism of the oxygen evolution reaction over NiFe-layered double hydroxides is investigated using first-principles DFT + U calculations. We consider three possible reaction pathways for O2 evolution. Our calculations show that O2 evolution from the OH*–OH* species has high energy barrier and from OOH* species have a little high energy barrier. Finally, we discover that O2 can easily release from OO* species.
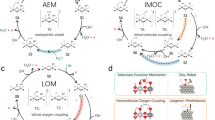
Graphical Abstract
The mechanism of oxygen evolution reaction (OER) over NiFe-layered double hydroxides was investigated using DFT + U method by First-principle. The present work considered two possible reaction pathways for O2 evolution. Our calculation suggested that O2 evolution from the OOH* species may be more favorable because of advantages in energy profile, oxygen adsorption, and overall energy barrier. In addition, density of states (DOS) and partial density of states (PDOSs) of NiFe-LDH and Ni(OH)2 showed that NiFe-LDH had a more stronger capability for electron transportation and higher activity than Ni(OH)2. Additionally, it was found that Bader charge of Ni had a large fluctuation in the former elementary steps, while in the later that of Fe had. The research suggested that the transitional metal Fe/Ni-based hydrotalcite was a suitable material for OER, for not only they had great activity and stability, but also they were widely used and comparatively cheap.









Similar content being viewed by others
References
Gray HB (2009) Nat Chem 1:7
Kudo A, Miseki Y (2009) Soc Rev 38:253
Lu YC, Xu Z, Gasteiger HA, Chen S, Hamad-Schifferli K, Shao-Horn Y (2010) J Am Chem Soc 132:12170
Walter MG, Warren EL, McKone JR, Boettcher SW, Mi Q, Santori EA, Lewis NS (2010) Chem Rev 110:6446
Cheng F, Chen J (2012) Chem Soc Rev 41:2172
Liang Y, Li Y, Wang H, Dai H (2013) J Am Chem Soc 135:2013
Wang H, Dai H (2013) Chem Soc Rev 42:3088
Hamdani M, Singh RN, Chartier P (2010) Int J Electrochem Sci 5:556
Oshikiri M, Boero M (2010) J Phys Chem B 110:9188
Yang J, Wang D, Zhou X, Li C (2013) Chem Eur J 19:1320
Kanan MW, Nocera DG (2008) Science 321:1072
Gong M, Li Y, Wang H, Liang Y, Wu JZ, Zhou J, Wang J, Regier T, Wei F, Dai H (2013) J Am Chem Soc 135:8452
Lee Y, Suntivich J, May KJ, Perry EE, Shao-Horn Y (2012) J Phys Chem Lett 3:399
Liu G, Han J, Zhou X, Huang L, Zhang F, Wang X, Ding C, Zheng X, Han H, Li C (2013) J Catal 307:148
Liu X, Wang F (2012) Coord Chem Rev 256:1115
Rossmeisl J, Qu ZW, Zhu H, Kroes GJ, Norskov JK (2007) J Electroanal Chem 607:83
Fang YH, Liu ZP (2010) J Am Chem Soc 132:18214
Dincă M, Surendranath Y, Nocera DG (2010) Proc Natl Acad Sci 10:10337
Singh A, Chang SL, Hocking RK, Bach U, Spiccia L (2013) Energy Environ Sci 6:579
Rahman G, Joo OS (2012) Int J Hydrogen Energy 37:13989
Gong M, Li Y, Wang H, Liang Y, Wu JZ, Zhou J, Wang J, Regier T, Wei F, Dai H (2013) ar**v preprint ar**v 1303:3308
Kim SJ, Lee Y, Lee DK, Lee JW, Kang J (2014) J Mater Chem A 2:4136
Zhang Y, Cui B, Zhao C, Lin H, Li J (2013) Phys Chem Chem Phys 15:7363
Chen X, Fu C, Wang Y, Yang W, Evans DG (2008) Biosens Bioelectron 24:356
Bolshak E, Abelló S, Montané D (2013) Int J Hydrogen Energy 38:5594
Sakaebe H, Uchino H, Azuma M, Shikano M, Higuchi S (1998) Solid State Ion 113:35
Bajdich M, García-Mota M, Vojvodic A, Nørskov JK, Bell AT (2013) J Am Chem Soc 135:13521
Kresse G, Furthmüller J (1996) Comput Mater Sci 6:15
Aguilera I, Palacios P, Wahnón P (2010) Sol Energy Mater Sol Cells 94:1903
Blöchl PE (1994) Phys Rev B 50:17953
Cheng MY, Hwang BJ (2007) Nanoscale Res Lett 2:28
Gao M, Sheng W, Zhuang Z, Fang Q, Gu S, Jiang J, Yan Y (2014) J Am Chem Soc 136:7077
Man IC, Su HY, Calle-Vallejo F, Hansen HA, Martínez JI, Inoglu NG, Kitchin J, Jaramillo TF, Norskov JK, Rossmeisl J (2011) ChemCatChem 3:1159
Kanan MW, Yano J, Surendranath Y, Dinca M, Yachandra VK, Nocera DG (2010) J Am Chem Soc 132:13692
Garcia-Mota M, Bajdich M, Viswanathan V, Vojvodic A, Bell AT, Nørskov JK (2012) J Phys Chem C 116:21077
Bediako DK, Lassalle-Kaiser B, Surendranath Y, Yano J, Yachandra VK, Nocera DG (2012) J Am Chem Soc 134:6801
Bediako DK, Surendranath Y, Nocera DG (2013) J Am Chem Soc 135:3662
McCrory CC, Jung S, Peters JC, Jaramillo TF (2013) J Am Chem Soc 135:16977
Li YF, Selloni A (2014) ACS Catal 4:1148
Prevot V, Forano C, Besse JP (2000) J Solid State Chem 153:301
Crepaldi EL, Valim JB (1998) Quim Nova 21:300
Oliver-Tolentino MA, Vázquez-Samperio J, Manzo-Robledo A, Gonzá lez-Huerta RDG, Flores-Moreno JL, Ramírez-Rosales D, Guzmán-Vargas A (2014) J Phys Chem C 118:22432
Lu Z, Xu W, Zhu W, Yang Q, Lei X, Liu J, Li Y, Sun X, Duan X (2014) Chem Commun 50:6479
Tronto J, Sanchez KC, Crepaldi EL, Naal Z, Klein SI, Valim JB (2004) J Phys Chem Solids 65:493
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865
Wasileski SA, Janik MJ (2008) Phys Chem Chem Phys 10:3613
Dudarev SL, Botton GA, Savrasov SY, Humphreys CJ, Sutton AP (1998) Phys Rev B 57:1505
Wang L, Maxisch T, Ceder G (2006) Phys Rev B 73:195107
Del Arco M, Malet P, Trujillano R, Rives V (1999) Chem Mater 11:624
Abelló S, Bolshak E, Montané D (2013) Appl Catal A Gen 450:261
Costa DG, Rocha AB, Diniz R, Souza WF, Chiaro SSX, Leitão AA (2010) J Phys Chem C 114:14133
Matsumoto Y, Sato E (1986) Mater Chem Phys 14:397
Trotochaud L, Young SL, Ranney JK, Boettcher SW (2014) J Am Chem Soc 136:6744
Garcia-Mota M, Bajdich M, Viswanathan V, Vojvodic A, Bell AT, Nørskov JK (2010) J Phys Chem C 16:21077
Chemelewski WD, Lee HC, Lin JF, Bard AJ, Mullins CB (2014) J Am Chem Soc 136:2843
Yuan L, Li Z, Yang J, Hou JG (2012) Phys Chem Chem Phys 14:8179
Zhou C, Zhang Q, Chen L, Han B, Ni G, Wu J, Garg D, Cheng H (2010) J Phys Chem C114:21405
Acknowledgments
This study was supported by the National Science Foundation of China (21103007), 863 Program (2012AA03A609), by the Youth Education Talent Plan of Bei**g (YETP0510), and by the Fundamental Research Funds for the Central Universities (YS1406). The study was also supported by “CHEMCLOUDCOMPUTING” of Bei**g University of Chemical Technology.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dong, Y., Zhang, P., Kou, Y. et al. A First-Principles Study of Oxygen Formation Over NiFe-Layered Double Hydroxides Surface. Catal Lett 145, 1541–1548 (2015). https://doi.org/10.1007/s10562-015-1561-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-015-1561-0