Abstract
Purpose
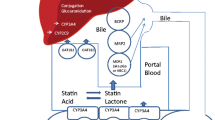
Hepatotoxicity has emerged as a major cause of statin treatment interruption. Although organic anion-transporting polypeptide 1B1 (SLCO1B1), multidrug resistance protein 1 (ABCB1), and breast cancer resistance protein (ABCG2) have been identified as transporters of statins, knowledge of their role in statin-associated hepatotoxicity remains limited. Therefore, we aimed to conduct a comprehensive analysis to elucidate the association between hepatotoxicity and SLCO1B1, ABCB1, and ABCG2 polymorphisms.
Methods
This study retrospectively analyzed prospectively collected samples. We selected 10 single nucleotide polymorphisms (SNPs) of SLCO1B1, 9 SNPs of ABCB1, and 12 SNPs of ABCG2. We developed two models for multivariable analyses (Model I: clinical factors only; Model II: both clinical and genetic factors), and the attributable risk (%) of variables in Model II was determined.
Results
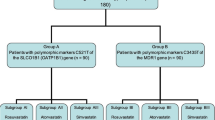
Among 851 patients, 66 (7.8%) developed hepatotoxicity. In Model I, lipophilic statins, atrial fibrillation (Afib), and diabetes mellitus showed a significant association with hepatotoxicity. In Model II, lipophilic statins and Afib, SLCO1B1 rs11045818 A allele, SLCO1B1 rs4149035 T allele, and ABCG2 rs2622629 TT genotype were associated with higher hepatotoxicity risk. Among them, the SLCO1B1 rs11045818 A allele exhibited the highest attributable risk (93.2%). The area under the receiver operating characteristic curve in Model I was 0.62 (95% CI: 0.55–0.69), and it was increased to 0.71 in Model II (95% CI: 0.64–0.77).
Conclusion
This study investigated the correlation between hepatotoxicity and polymorphisms of transporter genes in patients taking statins. The findings could help improve personalized treatments for patients receiving statin therapy.


Similar content being viewed by others
Data Availability
The datasets analyzed during the current study are presented as a supplementary file.
Code Availability
Not applicable.
References
Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–143. https://doi.org/10.1161/cir.0000000000000625.
Sizar O, Khare S, Jamil RT, Talati R. Statin Medications. https://www.ncbi.nlm.nih.gov/books/NBK430940/.
Efficacy. Safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393(10170):407–15. https://doi.org/10.1016/s0140-6736(18)31942-1.
Thompson PD, Panza G, Zaleski A, Taylor B. Statin-Associated Side effects. J Am Coll Cardiol. 2016;67(20):2395–410. https://doi.org/10.1016/j.jacc.2016.02.071.
Licata A, Giammanco A, Minissale MG, et al. Liver and statins: a critical Appraisal of the evidence. Curr Med Chem. 2018;25(42):5835–46. https://doi.org/10.2174/0929867325666180327095441.
Bhardwaj SS, Chalasani N. Lipid-lowering agents that cause drug-induced hepatotoxicity. Clin Liver Dis. 2007;11(3):597–613, vii. https://doi.org/10.1016/j.cld.2007.06.010.
Björnsson E, Jacobsen EI, Kalaitzakis E. Hepatotoxicity associated with statins: reports of idiosyncratic liver injury post-marketing. J Hepatol. 2012;56(2):374–80. https://doi.org/10.1016/j.jhep.2011.07.023.
Rocha KCE, Pereira BMV, Rodrigues AC. An update on efflux and uptake transporters as determinants of statin response. Expert Opin Drug Metab Toxicol. 2018;14(6):613–24. https://doi.org/10.1080/17425255.2018.1482276.
Turongkaravee S, Jittikoon J, Lukkunaprasit T, et al. A systematic review and meta-analysis of genotype-based and individualized data analysis of SLCO1B1 gene and statin-induced myopathy. Pharmacogenomics J. 2021;21(3):296–307. https://doi.org/10.1038/s41397-021-00208-w.
Kee PS, Chin PKL, Kennedy MA, Maggo SDS. Pharmacogenetics of Statin-Induced myotoxicity. Front Genet. 2020;11:575678. https://doi.org/10.3389/fgene.2020.575678.
Streja L, Packard CJ, Shepherd J, Cobbe S, Ford I. Factors affecting low-density lipoprotein and high-density lipoprotein cholesterol response to pravastatin in the West of Scotland Coronary Prevention Study (WOSCOPS). Am J Cardiol. 2002;90(7):731–6. https://doi.org/10.1016/s0002-9149(02)02599-7.
Qu KK, Zhang CN, Dong LX, et al. Association of ABCB1 polymorphisms with lipid homeostasis and liver injury response to atorvastatin in the Chinese population. Can J Physiol Pharmacol. 2020;98(1):15–22. https://doi.org/10.1139/cjpp-2019-0339.
Mirošević Skvrce N, Macolić Šarinić V, Šimić I, et al. ABCG2 gene polymorphisms as risk factors for atorvastatin adverse reactions: a case-control study. Pharmacogenomics. 2015;16(8):803–15. https://doi.org/10.2217/pgs.15.47.
Merćep I, Radman I, Trkulja V, et al. Loss of function polymorphisms in SLCO1B1 (c.521T > C, rs4149056) and ABCG2 (c.421C > A, rs2231142) genes are associated with adverse events of rosuvastatin: a case-control study. Eur J Clin Pharmacol. 2022;78(2):227–36. https://doi.org/10.1007/s00228-021-03233-7.
Yow HY, Hamzah S, Abdul Rahim N, Suppiah V. Pharmacogenomics of response to statin treatment and susceptibility to statin-induced adverse drug reactions in asians: a sco** review. Asian Biomed (Res Rev News). 2023;17(3):95–114. https://doi.org/10.2478/abm-2023-0050.
Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES. National Institutes of Health. 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Accessed 03 Apr 2023.
Meurer L, Cohen SM. Drug-Induced Liver Injury from statins. Clin Liver Dis. 2020;24(1):107–19. https://doi.org/10.1016/j.cld.2019.09.007.
McKenney JM, Davidson MH, Jacobson TA, Guyton JR. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97(8a):c89–94. https://doi.org/10.1016/j.amjcard.2006.02.030.
Boivin AA, Cardinal H, Barama A, et al. Organic anion transporting polypeptide 1B1 (OATP1B1) and OATP1B3: genetic variability and haplotype analysis in white canadians. Drug Metab Pharmacokinet. 2010;25(5):508–15. https://doi.org/10.2133/dmpk.dmpk-10-sh-046.
Nguyen HH, Nguyen CTT, Mai TNP, Huong PT. Associations between four polymorphisms of the SLCO1B1 and effectiveness of the statins: a meta-analysis. Pharmacogenet Genomics. 2023;33(4):65–78. https://doi.org/10.1097/fpc.0000000000000490.
Peters BJ, Rodin AS, Klungel OH, et al. Pharmacogenetic interactions between ABCB1 and SLCO1B1 tagging SNPs and the effectiveness of statins in the prevention of myocardial infarction. Pharmacogenomics. 2010;11(8):1065–76. https://doi.org/10.2217/pgs.10.81.
Yoon HY, Song TJ, Yee J, Park J, Gwak HS. Association between Genetic Polymorphisms and bleeding in patients on direct oral anticoagulants. Pharmaceutics. 2022;14(9). https://doi.org/10.3390/pharmaceutics14091889.
Levran O, O’Hara K, Peles E, et al. ABCB1 (MDR1) genetic variants are associated with methadone doses required for effective treatment of heroin dependence. Hum Mol Genet. 2008;17(14):2219–27. https://doi.org/10.1093/hmg/ddn122.
Kim H, Song TJ, Yee J, et al. ABCG2 gene polymorphisms may affect the bleeding risk in patients on Apixaban and Rivaroxaban. Drug Des Devel Ther. 2023;17:2513–22. https://doi.org/10.2147/dddt.S417096.
Boocock J, Leask M, Okada Y, et al. Genomic dissection of 43 serum urate-associated loci provides multiple insights into molecular mechanisms of urate control. Hum Mol Genet. 2020;29(6):923–43. https://doi.org/10.1093/hmg/ddaa013.
Ye J, Zeng Z, Chen Y, et al. Examining an Association of Single Nucleotide Polymorphisms with Hyperuricemia in Chinese Flight attendants. Pharmgenomics Pers Med. 2022;15:589–602. https://doi.org/10.2147/pgpm.S364206.
Kiyotani K, Mushiroda T, Imamura CK, et al. Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients. J Clin Oncol. 2010;28(8):1287–93. https://doi.org/10.1200/jco.2009.25.7246.
Ward LD, Kellis M. HaploReg v4: systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic Acids Res. 2016;44(D1):D877–81. https://doi.org/10.1093/nar/gkv1340.
Sayers EW, Bolton EE, Brister JR, et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2022;50(D1):D20–6. https://doi.org/10.1093/nar/gkab1112.
Averbukh LD, Turshudzhyan A, Wu DC, Wu GY. Statin-induced Liver Injury patterns: a clinical review. J Clin Transl Hepatol. 2022;10(3):543–52. https://doi.org/10.14218/jcth.2021.00271.
Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011;63(1):157–81. https://doi.org/10.1124/pr.110.002857.
** S, Li X, Fan Y, et al. Association between genetic polymorphisms of SLCO1B1 and susceptibility to methimazole-induced liver injury. Basic Clin Pharmacol Toxicol. 2019;125(6):508–17. https://doi.org/10.1111/bcpt.13284.
Alhawari H, Jarrar Y, AlKhatib MA, et al. The Association of 3-Hydroxy-3-Methylglutaryl-CoA Reductase, apolipoprotein E, and Solute Carrier Organic Anion Genetic Variants with Atorvastatin Response among Jordanian patients with type 2 diabetes. Life (Basel). 2020;10(10). https://doi.org/10.3390/life10100232.
Giannakopoulou E, Ragia G, Kolovou V, et al. No impact of SLCO1B1 521T > C, 388A > G and 411G > A polymorphisms on response to statin therapy in the Greek population. Mol Biol Rep. 2014;41(7):4631–8. https://doi.org/10.1007/s11033-014-3334-z.
Ramsey LB, Gong L, Lee SB, et al. PharmVar GeneFocus: SLCO1B1. Clin Pharmacol Ther. 2023;113(4):782–93. https://doi.org/10.1002/cpt.2705.
Mykkänen AJH, Taskinen S, Neuvonen M, et al. Genomewide Association Study of Simvastatin Pharmacokinetics. Clin Pharmacol Ther. 2022;112(3):676–86. https://doi.org/10.1002/cpt.2674.
Lopez-Lopez E, Ballesteros J, Piñan MA, et al. Polymorphisms in the methotrexate transport pathway: a new tool for MTX plasma level prediction in pediatric acute lymphoblastic leukemia. Pharmacogenet Genomics. 2013;23(2):53–61. https://doi.org/10.1097/FPC.0b013e32835c3b24.
Marciante KD, Durda JP, Heckbert SR, et al. Cerivastatin, genetic variants, and the risk of rhabdomyolysis. Pharmacogenet Genomics. 2011;21(5):280–8. https://doi.org/10.1097/FPC.0b013e328343dd7d.
Hernesniemi JA, Lyytikäinen LP, Oksala N, et al. Predicting sudden cardiac death using common genetic risk variants for coronary artery disease. Eur Heart J. 2015;36(26):1669–75. https://doi.org/10.1093/eurheartj/ehv106.
Kiander W, Sjöstedt N, Manninen R, et al. Functional in vitro characterization of SLCO1B1 variants and simulation of the clinical pharmacokinetic impact of impaired OATP1B1 function. Eur J Pharm Sci. 2022;176:106246. https://doi.org/10.1016/j.ejps.2022.106246.
Krishnamurthy P, Schuetz JD. Role of ABCG2/BCRP in biology and medicine. Annu Rev Pharmacol Toxicol. 2006;46:381–410. https://doi.org/10.1146/annurev.pharmtox.46.120604.141238.
Battle A, Brown CD, Engelhardt BE, Montgomery SB. Genetic effects on gene expression across human tissues. Nature. 2017;550(7675):204–13. https://doi.org/10.1038/nature24277.
Bytyçi I, Bajraktari G, Bhatt DL, et al. Hydrophilic vs lipophilic statins in coronary artery disease: a meta-analysis of randomized controlled trials. J Clin Lipidol. 2017;11(3):624–37. https://doi.org/10.1016/j.jacl.2017.03.003.
Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol. 2005;19(1):117–25. https://doi.org/10.1111/j.1472-8206.2004.00299.x.
Fong CW. Statins in therapy: understanding their hydrophilicity, lipophilicity, binding to 3-hydroxy-3-methylglutaryl-CoA reductase, ability to cross the blood brain barrier and metabolic stability based on electrostatic molecular orbital studies. Eur J Med Chem. 2014;85:661–74. https://doi.org/10.1016/j.ejmech.2014.08.037.
Karahalil B, Hare E, Koç G, et al. Hepatotoxicity associated with statins. Arh Hig Rada Toksikol. 2017;68(4):254–60. https://doi.org/10.1515/aiht-2017-68-2994.
Makar GA, Weiner MG, Kimmel SE, et al. Incidence and prevalence of abnormal liver associated enzymes in patients with atrial fibrillation in a routine clinical care population. Pharmacoepidemiol Drug Saf. 2008;17(1):43–51. https://doi.org/10.1002/pds.1514.
Miyamoto R, Nagao K, Matsuto K, et al. Relationship between atrial fibrillation and a liver fibrogenesis marker in patients with acute heart failure. Int J Cardiol. 2023;374:51–7. https://doi.org/10.1016/j.ijcard.2023.01.001.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (grant number: NRF-2023R1A2C1007463).
Author information
Authors and Affiliations
Contributions
All the authors have made substantial contributions to the conception of the study. SAC, JSK, TJS, and HSG contributed to designing the study. YC and TJS contributed to material preparation and YAP, DHL, MP and JY contributed to data collection. SAC and JSK performed data analysis and interpretation. SAC and JSK contributed to the drafting of the manuscript. TJS and HSG contributed to the critical revision of the manuscript. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Ewha Womans University Seoul Hospital and Ewha Womans University Mokdong Hospital (IRB number: 2020-11-014 and 2021-02-026, respectively).
Consent for Publication
All authors approved the final manuscript and the submission to this journal.
Consent to Participate
Written informed consent was obtained from all patients.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choi, SA., Kim, J.S., Park, YA. et al. Transporter Genes and statin-induced Hepatotoxicity. Cardiovasc Drugs Ther (2024). https://doi.org/10.1007/s10557-024-07580-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s10557-024-07580-2