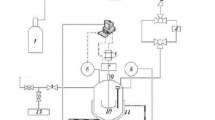
The growth features of a hydrate film at the interface between liquid carbon dioxide and pure water and sodium dodecyl sulfate solution were studied. It was found in all cases that in a Teflon cell a hydrate film is formed on the contact surface of the liquid phases, after which no visible changes are observed for an indefinitely long time. In experiments with pure water in a steel cell after the water-CO2 surface is covered with hydrate the hydrate film is slowly deformed as a result of growth along the line of contact between the hydrate and the cell wall. In experiments with a solution of sodium dodecyl sulfate the deformation of the hydrate film is much more pronounced, and the degree of conversion of the water into hydrate is increased significantly.





Similar content being viewed by others
References
A. Yu. Manakov, A. S. Stoporev, Uspekhi Khimii, 90(5), 566-600 (2021).
L. P. Liu, Z. Sun, L. Zhang, et. al., Acta Geol. Sin. Engl., 93(3), 731-755 (2019).
J. Carroll, Natural Gas Hydrates. A Guide for Engineers [Russian translation] (Eds. A. N. Zolotous, M. Ya. Bchinskii), ZAO, Moscow (2007), 316 pp..
H. P. Veluswamy, A. Kumar, Y. Seo, et. al., Appl. Energy, 216, 262-285 (2018).
C.-G. Xu, Y.-S. Yu, W.-J. **e, et. al., Appl. Energy, 255, 113791 (2019).
M. S. Sergeeva, A. N. Petukhov, D. N. Shablykin, et al., Zhurnal Fizicheskoi Khimii, 96(1), 39-46 (2022).
J. Luo, Y. **e, M. Z. Hou, et. al., Energy Reviews, 2(1), 100016 (2023).
M. Bui, C. S. Adjiman, A. Bardow, et. al., Energy Environ. Sci., 11, 1062-1176 (2018).
S. Nanda, S. N. Reddy, S. K. Mitra, et. al., Energy Sci. Eng., 4, 99-122 (2016).
X. Wang, F. Zhang, W. Lipinski, Appl. Energy, 269, 114928 (2020).
B. Tohidi, J. Yang, M. Salehabadi, et. al., Environ. Sci. Technol., 44, 1509-1514 (2010).
M. M. Cote, J. F. Wright, Preliminary Assessment of the Geological Potential for Sequestration of CO2 as Gas Hydrate in the Alberta Portion of the Western Canada Sedimentary Basin: Natural Resources Canada, Society of Petroleum Engineers (2013).
X. M. Zhang, J. P. Li, J. X. Wang, et. al., Int. J. Green Energ., 18(7), 1-10 (2021).
C. Y. Sun, B. Z. Peng, A. Dandekar, et. al., Ann. Rep. Prog. Chem., Sect. C: Phys. Chem., 106, 77-100 (2010).
V. A. Vlasov, Heat and Mass Transfer, 55, 3537-3545 (2019).
T. Uchida, T. Ebinuma, J. Kawabata, et. al., J. Cryst. Growth, 204(3), 348-356 (1999).
T. Uchida, I. Y. Ikeda, S. Takeya, et. al., J. Cryst. Growth, 237-239, 383-387 (2002).
B. Z. Peng, A. Dandekar, C. Y. Sun, et. al., J. Phys. Chem. B, 111(43), 12485-12493 (2007).
D. Daniel-David, F. Guerton., C. Dicharry, et. al., Chem. Eng. Sci., 132(18), 118-127 (2015).
V. P. Melnikov, A. N. Nesterov, A. M. Reshetnikov, et. al., Chem. Eng. Sci., 66, 73-77 (2011).
T. P. Adamova, S. S. Skiba, A. Y. Manakov, S. Y. Misyura, Chinese J. Chem. Eng., 56, 266-272 (2023).
Y. Abe, X. Ma, T. Yanai, et. al., AIChE J, 62(11), 4078-4089 (2016).
O. B. Kutergin, V. P. Melnikov, A. N. Nesterov, Doklady Akademii Nauk, 323(3). 549-553 (1992).
Q. Nasir, H. Suleman, Y. A. Elsheikh, J. Nat. Gas Sci. Eng., 76, 103211 (2020).
E. Chaturvedi, S. Laik, A. Mandal, Chinese J. Chem. Eng., 32, 1-16 (2021).
Z. **a, Q. Zhao, Z. Chen, et. al., J. Nat. Gas Sci. Eng., 101, 104528 (2022).
N. S. Molokitina, A. N. Nesterov, L. S. Podenko, et. al., Fuel, 235, 1400-1411 (2019).
A. N. Nesterov, A. M. Reshetnikov, Chem. Eng. J., 378, 122165 (2019).
A. Kumar, T. Sakpal, P. Linga, et. al., Fuel, 105, 664-671 (2013).
P. M. Naullage, A. A. Bertolazzo, V. Molinero, ACS Central Science, 5, 428-439 (2019).
D. A. Strukov, T. P. Adamova, A. Y. Manakov, Cryst. Growth Des., 23(1), 354-361 (2023).
A. S. Stoporev, T. P. Adamova, A. Y. Manakov, Cryst. Growth Des., 20(3). 1927-1934 (2020).
H. Liang, D. Guan, Y. Liu, et. al., J. Colloid Interf. Sci., 626, 1003-1014 (2022).
Acknowledgments
The investigation was supported by the Russian Science Foundation, grant No. 23-29-00830, https://rscf.ru/project/23-29-00830/.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 4, pp. 50–56, July – August, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sagidullin, A.K., Manakov, A.Y. Growth Features of Gas Hydrate Films at Interface of Liquid Carbon Dioxide with Water and Sodium Dodecyl Sulfate Solution in Teflon and Steel Cuvettes. Chem Technol Fuels Oils 59, 718–725 (2023). https://doi.org/10.1007/s10553-023-01575-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-023-01575-9