Abstract
Purpose
To evaluate the efficacy and safety of pegylated liposomal doxorubicin (PLD) in patients with human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer (MBC) heavily pretreated with anthracycline and taxanes.
Methods
In this single-arm, phase II study, patients with HER2-negative MBC previously treated with anthracycline and taxanes as second- to fifth chemotherapy received PLD (Duomeisu®, generic doxorubicin hydrochloride liposome) 40 mg/m2 every 4 weeks until disease progression, unacceptable toxicity, or completion of six cycles. Primary endpoint was progression-free survival (PFS). Secondary endpoints included overall survival (OS), objective response rate (ORR), disease control rate (DCR), clinical benefit rate (CBR), and safety.
Results
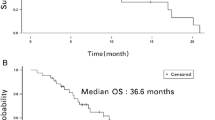
Of 44 enrolled patients (median age, 53.5 years; range, 34–69), 41 and 36 were evaluable for safety and efficacy, respectively. In total, 59.1% (26/44) of patients had ≥ 3 metastatic sites, 86.4% (38/44) had visceral disease, and 63.6% (28/44) had liver metastases. Median PFS was 3.7 months (95% confidence interval [CI] 3.3–4.1) and median OS was 15.0 months (95% CI 12.1–17.9). ORR, DCR, and CBR were 16.7%, 63.9%, and 36.1%, respectively. The most common adverse events (AEs) were leukopenia (53.7%), fatigue (46.3%), and neutropenia (41.5%), with no grade 4/5 AEs. The most common grade 3 AEs were neutropenia (7.3%) and fatigue (4.9%). Patients experienced palmar-plantar-erythrodysesthesia (24.4%, 2.4% grade 3), stomatitis (19.5%, 7.3% grade 2), and alopecia (7.3%). One patient displayed a left ventricular ejection fraction decline of 11.4% from baseline after five cycles of PLD therapy.
Conclusion
PLD (Duomeisu®) 40 mg/m2 every 4 weeks was effective and well-tolerated in patients with HER2-negative MBC heavily pretreated with anthracycline and taxanes, revealing a potentially viable treatment option for this population.
Trial registration Chinese Clinical Trial Registry: ChiCTR1900022568.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer has become the most commonly diagnosed cancer globally in 2020, surpassing lung cancer (11.4%), with an estimated 2,261,419 new cases (11.7%), and the fourth leading cause (6.9%) of cancer-related deaths worldwide, with an estimated 684,996 new deaths in 2020 [1]. Approximately 20–50% of patients with early breast cancer will eventually develop metastatic disease [2], and 5–10% of patients are initially diagnosed with metastatic disease [3]. Among patients with metastatic breast cancer (MBC), 62% are diagnosed with human epidermal growth factor receptor 2 (HER2)-negative disease [4]. Despite considerable advances in new treatments, MBC remains treatable but incurable, with the goal of treatment to prolong patients’ survival and improve their quality of life [5, 6].
During the past 70 years, the highest number of new drugs for breast cancer was approved [7]. Nevertheless, conventional anthracyclines (doxorubicin and epirubicin) and taxanes remain the cornerstones of breast cancer therapy regardless of the molecular subtype, whether in the neoadjuvant/adjuvant setting or metastatic setting[6, 8,39, 43].
This study had several limitations. First, this study had a single-center, open-label design with no control arm. Second, the implementation of the study took longer than originally planned. The slow enrollment of patients was aggravated by the COVID-19 pandemic. Third, this was an exploratory trial with small sample sizes.
Conclusion
In summary, our study demonstrated that PLD (Duomeisu®) monotherapy at 40 mg/m2 every 4 weeks was effective and safe in patients with HER2-negative MBC heavily pretreated with conventional anthracycline and taxanes, indicating that this treatment is a viable treatment option for this population.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ADC:
-
Antibody–drug conjugate
- AE:
-
Adverse event
- AIDS:
-
Acquired immune deficiency syndrome
- CBR:
-
Clinical benefit rate
- CI:
-
Confidence interval
- CR:
-
Complete response
- DCR:
-
Disease control rate
- HER2:
-
Human epidermal growth factor receptor 2
- HR:
-
Hormone receptor
- LVEF:
-
Left ventricular ejection fraction
- MBC:
-
Metastatic breast cancer
- ORR:
-
Objective response rates
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- PLD:
-
Pegylated liposomal doxorubicin
- PPE:
-
Palmar-plantar erythrodysesthesia
- PR:
-
Partial response
- RECIST:
-
Response Evaluation Criteria for Solid Tumor
- SD:
-
Stable disease
- SG:
-
Sacituzumab govitecan
- T-DXd:
-
Trastuzumab deruxtecan
- Trop-2:
-
Trophoblast cell surface antigen-2
- ULN:
-
Upper limit of normal
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA 71:209–249. https://doi.org/10.3322/caac.21660
Lu J, Steeg PS, Price JE, Krishnamurthy S, Mani SA, Reuben J et al (2009) Breast cancer metastasis: challenges and opportunities. Cancer Res 69:4951–4953. https://doi.org/10.1158/0008-5472.Can-09-0099
Cardoso F, Spence D, Mertz S, Corneliussen-James D, Sabelko K, Gralow J et al (2018) Global analysis of advanced/metastatic breast cancer: decade report (2005–2015). Breast 39:131–138. https://doi.org/10.1016/j.breast.2018.03.002
Nersesyan K, Robinson D, Pomerantz D (2014) Comparison of epidemiology and drug treatment in HER2 negative metastatic breast cancer (MBC) in EU5. Value Health 17:A619. https://doi.org/10.1016/j.jval.2014.08.2189
Caswell-** JL, Plevritis SK, Tian L, Cadham CJ, Xu C, Stout NK et al (2018) Change in survival in metastatic breast cancer with treatment advances: meta-analysis and systematic review. JNCI Cancer Spectr 2:062. https://doi.org/10.1093/jncics/pky062
Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F et al (2020) 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 31:1623–1649. https://doi.org/10.1016/j.annonc.2020.09.010
Leo CP, Leo C, Szucs TD (2020) Breast cancer drug approvals by the US FDA from 1949 to 2018. Nat Rev Drug Discov 19:11. https://doi.org/10.1038/d41573-019-00201-w
Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M et al (2012) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379:432–444. https://doi.org/10.1016/s0140-6736(11)61625-5
Fujii T, Le Du F, **ao L, Kogawa T, Barcenas CH, Alvarez RH et al (2015) Effectiveness of an adjuvant chemotherapy regimen for early-stage breast cancer: a systematic review and network meta-analysis. JAMA Oncol 1:1311–1318. https://doi.org/10.1001/jamaoncol.2015.3062
Ditsch N, Kolberg-Liedtke C, Friedrich M, Jackisch C, Albert US, Banys-Paluchowski M et al (2021) AGO Recommendations for the diagnosis and treatment of patients with early breast cancer: update 2021. Breast Care (Basel) 16:214–227. https://doi.org/10.1159/000516419
Gennari A, André F, Barrios CH, Cortés J, de Azambuja E, DeMichele A et al (2021) ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol 32:1475–1495. https://doi.org/10.1016/j.annonc.2021.09.019
Gennari A, André F, Barrios CH, Cortés J, de Azambuja E, DeMichele A et al (2021) ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. https://doi.org/10.1016/j.annonc.2021.09.019
Denduluri N, Somerfield MR, Eisen A, Holloway JN, Hurria A, King TA et al (2016) Selection of optimal adjuvant chemotherapy regimens for human epidermal growth factor receptor 2 (HER2)-negative and adjuvant targeted therapy for HER2-positive breast cancers: an American Society of Clinical Oncology guideline adaptation of the cancer care Ontario clinical practice guideline. J Clin Oncol 34:2416–2427. https://doi.org/10.1200/jco.2016.67.0182
Senkus E, Kyriakides S, Penault-Llorca F, Poortmans P, Thompson A, Zackrisson S et al (2013) Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24(Suppl 6):vi7–vi23. https://doi.org/10.1093/annonc/mdt284
Lüftner D, Bauerfeind I, Braun M, Brucker SY, Fasching PA, Felberbaum R et al (2019) Treatment of early breast cancer patients: evidence, controversies, consensus: focusing on systemic therapy—German experts’ opinions for the 16th International St. Gallen Consensus Conference (Vienna 2019). Breast Care (Basel) 14:315–324. https://doi.org/10.1159/000502603
Singal PK, Iliskovic N (1998) Doxorubicin-induced cardiomyopathy. N Engl J Med 339:900–905. https://doi.org/10.1056/nejm199809243391307
Ryberg M, Nielsen D, Cortese G, Nielsen G, Skovsgaard T, Andersen PK (2008) New insight into epirubicin cardiac toxicity: competing risks analysis of 1097 breast cancer patients. J Natl Cancer Inst 100:1058–1067. https://doi.org/10.1093/jnci/djn206
Barrett-Lee PJ, Dixon JM, Farrell C, Jones A, Leonard R, Murray N et al (2009) Expert opinion on the use of anthracyclines in patients with advanced breast cancer at cardiac risk. Ann Oncol 20:816–827. https://doi.org/10.1093/annonc/mdn728
Gabizon AA, Patil Y, La-Beck NM (2016) New insights and evolving role of pegylated liposomal doxorubicin in cancer therapy. Drug Resist Update 29:90–106. https://doi.org/10.1016/j.drup.2016.10.003
Berry G, Billingham M, Alderman E, Richardson P, Torti F, Lum B et al (1998) The use of cardiac biopsy to demonstrate reduced cardiotoxicity in AIDS Kaposi’s sarcoma patients treated with pegylated liposomal doxorubicin. Ann Oncol 9:711–716. https://doi.org/10.1023/a:1008216430806
Gabizon A, Shmeeda H, Barenholz Y (2003) Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin Pharmacokinet 42:419–436. https://doi.org/10.2165/00003088-200342050-00002
O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A et al (2004) Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol 15:440–449. https://doi.org/10.1093/annonc/mdh097
Chia S, Clemons M, Martin LA, Rodgers A, Gelmon K, Pond GR et al (2006) Pegylated liposomal doxorubicin and trastuzumab in HER-2 overexpressing metastatic breast cancer: a multicenter phase II trial. J Clin Oncol 24:2773–2778. https://doi.org/10.1200/jco.2005.03.8331
Batist G, Ramakrishnan G, Rao CS, Chandrasekharan A, Gutheil J, Guthrie T et al (2001) Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J Clin Oncol 19:1444–1454. https://doi.org/10.1200/jco.2001.19.5.1444
Harris L, Batist G, Belt R, Rovira D, Navari R, Azarnia N et al (2002) Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer 94:25–36. https://doi.org/10.1002/cncr.10201
Adamo V, Lorusso V, Rossello R, Adamo B, Ferraro G, Lorusso D et al (2008) Pegylated liposomal doxorubicin and gemcitabine in the front-line treatment of recurrent/metastatic breast cancer: a multicentre phase II study. Br J Cancer 98:1916–1921. https://doi.org/10.1038/sj.bjc.6604409
Yardley DA, Burris HA 3rd, Spigel DR, Clark BL, Vazquez E, Shipley D et al (2009) A phase II randomized crossover study of liposomal doxorubicin versus weekly docetaxel in the first-line treatment of women with metastatic breast cancer. Clin Breast Cancer 9:247–252. https://doi.org/10.3816/CBC.2009.n.042
Martín M, Sánchez-Rovira P, Muñoz M, Baena-Cañada JM, Mel JR, Margeli M et al (2011) Pegylated liposomal doxorubicin in combination with cyclophosphamide and trastuzumab in HER2-positive metastatic breast cancer patients: efficacy and cardiac safety from the GEICAM/2004-05 study. Ann Oncol 22:2591–2596. https://doi.org/10.1093/annonc/mdr024
Smorenburg CH, de Groot SM, van Leeuwen-Stok AE, Hamaker ME, Wymenga AN, de Graaf H et al (2014) A randomized phase III study comparing pegylated liposomal doxorubicin with capecitabine as first-line chemotherapy in elderly patients with metastatic breast cancer: results of the OMEGA study of the Dutch Breast Cancer Research Group BOOG. Ann Oncol 25:599–605. https://doi.org/10.1093/annonc/mdt588
Harbeck N, Saupe S, Jäger E, Schmidt M, Kreienberg R, Müller L et al (2017) A randomized phase III study evaluating pegylated liposomal doxorubicin versus capecitabine as first-line therapy for metastatic breast cancer: results of the PELICAN study. Breast Cancer Res Treat 161:63–72. https://doi.org/10.1007/s10549-016-4033-3
Keller AM, Mennel RG, Georgoulias VA, Nabholtz JM, Erazo A, Lluch A et al (2004) Randomized phase III trial of pegylated liposomal doxorubicin versus vinorelbine or mitomycin C plus vinblastine in women with taxane-refractory advanced breast cancer. J Clin Oncol 22:3893–3901. https://doi.org/10.1200/jco.2004.08.157
Martin M, García-Donas J, Casado A, de la Gándara I, Pérez-Segura P, García-Saenz JA et al (2004) Phase II study of pegylated liposomal doxorubicin plus vinorelbine in breast cancer with previous anthracycline exposure. Clin Breast Cancer 5:353–357. https://doi.org/10.3816/cbc.2004.n.041
Al-Batran SE, Bischoff J, von Minckwitz G, Atmaca A, Kleeberg U, Meuthen I et al (2006) The clinical benefit of pegylated liposomal doxorubicin in patients with metastatic breast cancer previously treated with conventional anthracyclines: a multicentre phase II trial. Br J Cancer 94:1615–1620. https://doi.org/10.1038/sj.bjc.6603158
Ardavanis A, Mavroudis D, Kalbakis K, Malamos N, Syrigos K, Vamvakas L et al (2006) Pegylated liposomal doxorubicin in combination with vinorelbine as salvage treatment in pretreated patients with advanced breast cancer: a multicentre phase II study. Cancer Chemother Pharmacol 58:742–748. https://doi.org/10.1007/s00280-006-0236-3
Chow LW, Yip AY, Lang BH (2007) A phase II trial of vinorelbine and pegylated liposomal doxorubicin in patients with pretreated metastatic breast cancer. Am J Clin Oncol 30:133–138. https://doi.org/10.1097/01.coc.0000251400.47711.fe
Rau KM, Lin YC, Chen YY, Chen JS, Lee KD, Wang CH et al (2015) Pegylated liposomal doxorubicin (Lipo-Dox®) combined with cyclophosphamide and 5-fluorouracil is effective and safe as salvage chemotherapy in taxane-treated metastatic breast cancer: an open-label, multi-center, non-comparative phase II study. BMC Cancer 15:423. https://doi.org/10.1186/s12885-015-1433-4
Martin-Romano P, Baraibar I, Espinós J, Legaspi J, López-Picazo JM, Aramendía JM et al (2018) Combination of pegylated liposomal doxorubicin plus gemcitabine in heavily pretreated metastatic breast cancer patients: Long-term results from a single institution experience. Breast J 24:473–479. https://doi.org/10.1111/tbj.12975
Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH et al (2020) Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 18:452–478. https://doi.org/10.6004/jnccn.2020.0016
Al-Batran SE, Meerpohl HG, von Minckwitz G, Atmaca A, Kleeberg U, Harbeck N et al (2006) Reduced incidence of severe palmar-plantar erythrodysesthesia and mucositis in a prospective multicenter phase II trial with pegylated liposomal doxorubicin at 40 mg/m2 every 4 weeks in previously treated patients with metastatic breast cancer. Oncology 70:141–146. https://doi.org/10.1159/000093005
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Harbeck N, Gnant M (2017) Breast cancer. Lancet 389:1134–1150. https://doi.org/10.1016/s0140-6736(16)31891-8
Al-Batran SE, Güntner M, Pauligk C, Scholz M, Chen R, Beiss B et al (2010) Anthracycline rechallenge using pegylated liposomal doxorubicin in patients with metastatic breast cancer: a pooled analysis using individual data from four prospective trials. Br J Cancer 103:1518–1523. https://doi.org/10.1038/sj.bjc.6605961
Fiegl M, Mlineritsch B, Hubalek M, Bartsch R, Pluschnig U, Steger GG (2011) Single-agent pegylated liposomal doxorubicin (PLD) in the treatment of metastatic breast cancer: results of an Austrian observational trial. BMC Cancer 11:373. https://doi.org/10.1186/1471-2407-11-373
Ji Y, Zhang X, Liu J, Chen Y, Meng M, Li C et al (2020) Direct quantitation of free, encapsulated, total doxorubicin and doxorubicinol in stabilized frozen human plasma to support a BE study of liposomal doxorubicin. J Pharm Biomed Anal 189:113388. https://doi.org/10.1016/j.jpba.2020.113388
Collea RP, Kruter FW, Cantrell JE, George TK, Kruger S, Favret AM et al (2012) Pegylated liposomal doxorubicin plus carboplatin in patients with metastatic breast cancer: a phase II study. Ann Oncol 23:2599–2605. https://doi.org/10.1093/annonc/mds052
Cortes J, O’Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K et al (2011) Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 377:914–923. https://doi.org/10.1016/s0140-6736(11)60070-6
Kaufman PA, Awada A, Twelves C, Yelle L, Perez EA, Velikova G et al (2015) Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 33:594–601. https://doi.org/10.1200/jco.2013.52.4892
Yuan P, Hu X, Sun T, Li W, Zhang Q, Cui S et al (2019) Eribulin mesilate versus vinorelbine in women with locally recurrent or metastatic breast cancer: a randomised clinical trial. Eur J Cancer 112:57–65. https://doi.org/10.1016/j.ejca.2019.02.002
Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E et al (2022) Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 387:9–20. https://doi.org/10.1056/NEJMoa2203690
Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M et al (2021) Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med 384:1529–1541. https://doi.org/10.1056/NEJMoa2028485
Rugo HS, Bardia A, Marmé F, Cortes J, Schmid P, Loirat D et al (2022) Sacituzumab govitecan in hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 40:3365–3376. https://doi.org/10.1200/jco.22.01002
Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N et al (2016) DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res 22:5097–5108. https://doi.org/10.1158/1078-0432.Ccr-15-2822
Goldenberg DM, Cardillo TM, Govindan SV, Rossi EA, Sharkey RM (2015) Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget 6:22496–22512. https://doi.org/10.18632/oncotarget.4318
Bardia A, Tolaney SM, Punie K, Loirat D, Oliveira M, Kalinsky K et al (2021) Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol 32:1148–1156. https://doi.org/10.1016/j.annonc.2021.06.002
Health Commission Of The People’s Republic Of China N (2022) National guidelines for diagnosis and treatment of breast cancer 2022 in China (English version). Chin J Cancer Res 34:151–175. https://doi.org/10.21147/j.issn.1000-9604.2022.03.02
Acknowledgements
We thank the participating patients and their families for their contributions to the study. We also thank Joe Barber Jr., PhD., from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript. This research was designed and led by the study investigators. Shijiazhuang Pharmaceutical Group Ouyi Pharmaceutical Co. Ltd (CSPC) provided some part of the study drug for this trial.
Author information
Authors and Affiliations
Contributions
HJ and HL contributed to the study conception and design. All authors were involved in the acquisition of data. The first draft of the manuscript was written by HJ, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
HPL received some part of the study drug for this trial from Shijiazhuang Pharmaceutical Group Ouyi Pharmaceutical Co. Ltd. All other authors declare that they have no potential conflicts of interest to disclose.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Peking University Cancer Hospital (ID:2017YJZ08).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Patients signed an informed consent form regarding the publication of their data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, H., Li, H., Song, G. et al. Pegylated liposomal doxorubicin (Duomeisu®) monotherapy in patients with HER2-negative metastatic breast cancer heavily pretreated with anthracycline and taxanes: a single-arm, phase II study. Breast Cancer Res Treat 199, 67–79 (2023). https://doi.org/10.1007/s10549-023-06894-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-06894-3