Abstract
Purpose
The estrogen receptor (ER) is involved in control of progesterone receptor (PgR) expression and lack of PgR may be also a surrogate of altered growth factor signaling. The aim of this study was therefore to investigate PgR expression as predictive factor for response to neoadjuvant therapy and long-term outcome.
Methods
Five thousand and six hundred and thirteen patients with primary breast cancer and positive ER expression from ten German neoadjuvant trials of anthracycline and taxane-based chemotherapy were included. Pathologic complete response (pCR), disease-free survival (DFS), distant disease-free survival (DDFS), overall survival (OS), and local recurrence-free survival (LRFS) were compared according to PgR expression.
Results
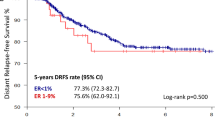
The lack of PgR expression (1172 patients) was associated with grade 3 (38.4 vs. 26.3%; p < 0.001), nodal involvement (>cN2) (6.8% vs. 4.7%; p = 0.004), and HER2 positivity (36.2 vs. 22.3%; p < 0.001). pCR rates of PgR-negative tumors were higher in the entire cohort (13.8 vs. 7.5%; p < 0.001) and in the HER2-negative subgroup (11.2 vs. 5.8%; p < 0.001). In multivariable logistic regression, PgR negativity was an independent predictive factor for pCR overall (OR 1.76; p < 0.001) and in the HER2-negative patients (OR 1.99; p < 0.001). Patients with PgR-negative disease had significantly worse outcome (p < 0.001, respectively). Multivariable Cox regression analysis revealed that PgR was an independent prognostic factor for DFS, OS, DDFS, and LRFS.
Conclusion
ER-positive/PgR-negative breast carcinomas are associated with higher response but also worse long-term outcome after neoadjuvant therapy. PgR negativity is an independent predictive factor for pCR after neoadjuvant chemotherapy in ER-positive HER2-negative breast cancer.





Similar content being viewed by others
References
Silva CM, Shupnik MA (2007) Integration of steroid and growth factor pathways in breast cancer: focus on signal transducers and activators of transcription and their potential role in resistance. Mol Endocrinol 21:1499–1512. doi:10.1210/me.2007-0109
Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E et al (1996) Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J 15:1292–1300
Dutertre M, Smith CL (2003) Ligand-independent interactions of p160/steroid receptor coactivators and CREB-binding protein (CBP) with estrogen receptor-alpha: regulation by phosphorylation sites in the A/B region depends on other receptor domains. Mol Endocrinol 17:1296–1314
Creighton CJ, van de Vijver MJ, Foekens JA, Klijn JG, Horlings HM et al (2009) Molecular profiles of progesterone receptor loss in human breast tumors. Breast Cancer Res Treat 114:287–299. doi:10.1007/s10549-008-0017-2
Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM (2003) Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol 21:1973–1979. doi:10.1200/JCO.2003.09.099
Dowsett M, Allred C, Knox J, Quinn E, Salter J, Wale C et al (2008) Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the arimidex, tamoxifen, alone or in combination trial. J Clin Oncol 26:1059–1065. doi:10.1200/JCO.2007.12.9437
Viale G, Regan MM, Maiorano E, Mastropasqua MG, Golouh R, Perin T et al (2008) Chemoendocrine compared with endocrine adjuvant therapies for node-negative breast cancer: predictive value of centrally reviewed expression of estrogen and progesterone receptors–International Breast Cancer Study Group. J Clin Oncol 26:1404–1410. doi:10.1200/JCO.2007.10.6393
Prat A, Cheang MC, Martin M, Parker JS, Carrasco E, Caballero R et al (2013) Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol 31:203–209. doi:10.1200/JCO.2012.43.4134
von Minckwitz G, Costa SD, Raab G, Blohmer JU, Eidtmann H, Hilfrich J et al (2001) Dose-dense doxorubicin, docetaxel, and granulocyte colony-stimulating factor support with or without tamoxifen as preoperative therapy in patients with operable carcinoma of the breast: a randomized, controlled, open phase IIb study. J Clin Oncol 19:3506–3515
von Minckwitz G, Raab G, Caputo A, Schutte M, Hilfrich J, Blohmer JU et al (2005) Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J Clin Oncol 23:2676–2685. doi:10.1200/JCO.2005.05.078
von Minckwitz G, Blohmer JU, Raab G, Lohr A, Gerber B, Heinrich G et al (2005) In vivo chemosensitivity-adapted preoperative chemotherapy in patients with early-stage breast cancer: the GEPARTRIO pilot study. Ann Oncol 16:56–63. doi:10.1093/annonc/mdi001
von Minckwitz G, Kummel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J et al (2008) Intensified neoadjuvant chemotherapy in early-responding breast cancer: phase III randomized GeparTrio study. J Natl Cancer Inst 100:552–562. doi:10.1093/jnci/djn089
von Minckwitz G, Kummel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J et al (2008) Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicin-cyclophosphamide in early nonresponsive breast cancer: phase III randomized GeparTrio trial. J Natl Cancer Inst 100:542–551. doi:10.1093/jnci/djn085
Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H et al (2010) Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol 28:2024–2031. doi:10.1200/JCO.2009.23.8451
Untch M, Mobus V, Kuhn W, Muck BR, Thomssen C, Bauerfeind I et al (2009) Intensive dose-dense compared with conventionally scheduled preoperative chemotherapy for high-risk primary breast cancer. J Clin Oncol 27:2938–2945. doi:10.1200/JCO.2008.20.3133
Untch M, Fasching PA, Konecny GE, von Koch F, Conrad U, Fett W et al (2011) PREPARE trial: a randomized phase III trial comparing preoperative, dose-dense, dose-intensified chemotherapy with epirubicin, paclitaxel and CMF versus a standard-dosed epirubicin/cyclophosphamide followed by paclitaxel ± darbepoetin alfa in primary breast cancer–results at the time of surgery. Ann Oncol 22:1988–1998. doi:10.1093/annonc/mdq709
Untch M, von Minckwitz G, Konecny GE, Conrad U, Fett W, Kurzeder C et al (2011) PREPARE trial: a randomized phase III trial comparing preoperative, dose-dense, dose-intensified chemotherapy with epirubicin, paclitaxel, and CMF versus a standard-dosed epirubicin-cyclophosphamide followed by paclitaxel with or without darbepoetin alfa in primary breast cancer–outcome on prognosis. Ann Oncol 22:1999–2006. doi:10.1093/annonc/mdq713
Untch M, Fasching PA, Konecny GE, Hasmuller S, Lebeau A, Kreienberg R et al (2011) Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol 29:3351–3357. doi:10.1200/JCO.2010.31.4930
von Minckwitz G, Untch M, Nuesch E, Loibl S, Kaufmann M, Kummel S et al (2011) Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials. Breast Cancer Res Treat 125:145–156. doi:10.1007/s10549-010-1228-x
von Minckwitz G, Eidtmann H, Rezai M, Fasching PA, Tesch H, Eggemann H et al (2012) Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med 366:299–309. doi:10.1056/NEJMoa1111065
Untch M, Loibl S, Bischoff J, Eidtmann H, Kaufmann M, Blohmer JU et al (2012) Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol 13:135–144. doi:10.1016/S1470-2045(11)70397-7
von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M et al (2014) Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 15:747–756. doi:10.1016/S1470-2045(14)70160-3
von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA et al (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30:1796–1804. doi:10.1200/JCO.2011.38.8595
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384:164–172. doi:10.1016/S0140-6736(13)62422-8
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA et al (2000) Molecular portraits of human breast tumours. Nature 406:747–752. doi:10.1038/35021093
Mohsin SK, Weiss H, Havighurst T, Clark GM et al (2004) Progesterone receptor by immunohistochemistry and clinical outcome in breast cancer: a validation study. Mod Pathol 17:1545–1554. doi:10.1038/modpathol.3800229
Dunnwald LK, Rossing MA, Li CI (2007) Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res 9:R6. doi:10.1186/bcr1639
Grann VR, Troxel AB, Zojwalla NJ, Jacobson JS, Hershman D, Neugut AI (2005) Hormone receptor status and survival in a population-based cohort of patients with breast carcinoma. Cancer 103:2241–2251. doi:10.1002/cncr.21030
Salmen J, Neugebauer J, Fasching PA, Haeberle L, Huober J, Wockel A et al (2014) Pooled analysis of the prognostic relevance of progesterone receptor status in five German cohort studies. Breast Cancer Res Treat 148:143–151. doi:10.1007/s10549-014-3130-4
Early Breast Cancer Trialists’ Collaborative G, Davies C, Godwin J, Gray R, Clarke M, Cutter D et al (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378:771–784. doi:10.1016/S0140-6736(11)60993-8
Dowsett M, Houghton J, Iden C, Salter J, Farndon J, A’Hern R et al (2006) Benefit from adjuvant tamoxifen therapy in primary breast cancer patients according oestrogen receptor, progesterone receptor, EGF receptor and HER2 status. Ann Oncol 17:818–826. doi:10.1093/annonc/mdl016
Viale G, Regan MM, Dell’Orto P, Mastropasqua MG, Maiorano E, Rasmussen BB et al (2011) Which patients benefit most from adjuvant aromatase inhibitors? Results using a composite measure of prognostic risk in the BIG 1-98 randomized trial. Ann Oncol 22:2201–2207. doi:10.1093/annonc/mdq738
Horwitz KB, McGuire WL (1978) Estrogen control of progesterone receptor in human breast cancer. Correlation with nuclear processing of estrogen receptor. J Biol Chem 253:2223–2228
Arpino G, Weiss H, Lee AV, Schiff R, De Placido S, Osborne CK et al (2005) Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst 97:1254–1261. doi:10.1093/jnci/dji249
Arpino G, Wiechmann L, Osborne CK, Schiff R (2008) Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev 29:217–233
Acknowledgements
We would like to thank all patients, investigators, and study personnel who supported the trials and the German Breast Group for support.
Funding
This study was supported by a Grant from the German Cancer Aid (Translational Oncology project 111536, TransLuminal-B).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Peter Fasching is a consultant for Novartis, Pfizer, and Roche and receives funding from Novartis. Claus Hanusch is a consultant for Roche, Pfizer Amgen, Asrazeneca, Celgene, and Novartis. Sherko Kuemmel is a consultant for Roche, Amgen, Novartis, Genomic Health, Cellgene, Tewa, and Dairdi-Saulay and receives funding from Roche. Frederik Marme acts as a consultant for Roche, AstraZeneca, Novartis, Amgen, and Genomic Health and receives remuneration from the aforementioned companies. All remaining authors declare that there exists no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
van Mackelenbergh, M.T., Denkert, C., Nekljudova, V. et al. Outcome after neoadjuvant chemotherapy in estrogen receptor-positive and progesterone receptor-negative breast cancer patients: a pooled analysis of individual patient data from ten prospectively randomized controlled neoadjuvant trials. Breast Cancer Res Treat 167, 59–71 (2018). https://doi.org/10.1007/s10549-017-4480-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4480-5